Example 13.11: Determination of Ka or Kb from pH
The pH of a 0.0516-M solution of nitrous acid, \(\ce{HNO2}\), is 2.34. What is its \(K_{\mathrm{a}}\)?\(\ce{HNO2(aq)}\)\(\ce{ + }\)\(\ce{H2O(l)}\)\(\ce{<=>}\)\(\ce{H3O+(aq)}\)\(\ce{ + }\)\(\ce{NO2-(aq)}\)\(\ce{ }\)
Solution
\(\mathrm{pH}\) \(= 2.34\)\([\ce{HNO2}]_{\mathrm{init}}\) \(= 0.0516\)
\(K_{\mathrm{a}}\) = ?
We determine an equilibrium constant starting with the initial concentrations of \(\ce{HNO2}\), \(\ce{H3O+}\), and \(\ce{NO2-}\) as well as one of the final concentrations, the concentration of hydronium ion at equilibrium. (Remember that pH is simply another way to express the concentration of hydronium ion.)
We can solve this problem with the following steps in which x is a change in concentration of a species in the reaction:
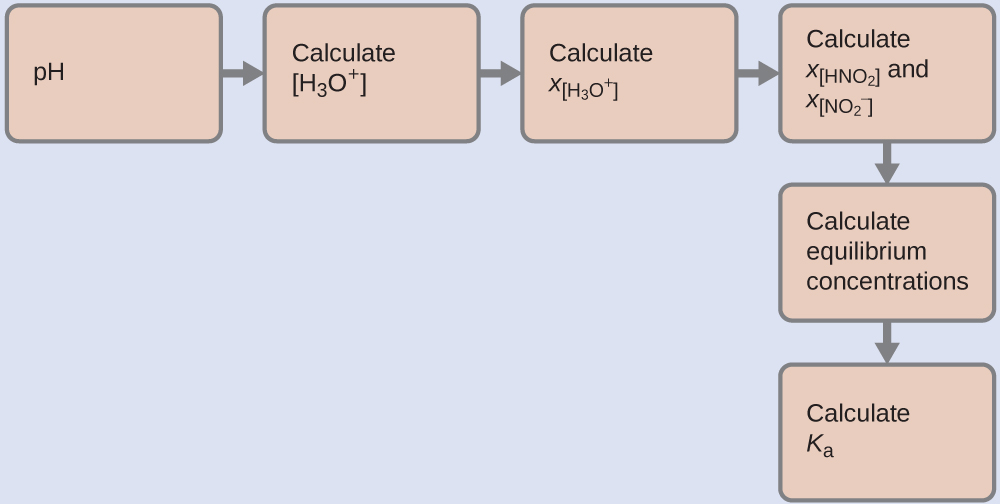
We can summarize the various concentrations and changes as shown here (the concentration of water does not appear in the expression for the equilibrium constant, so we do not need to consider its concentration):
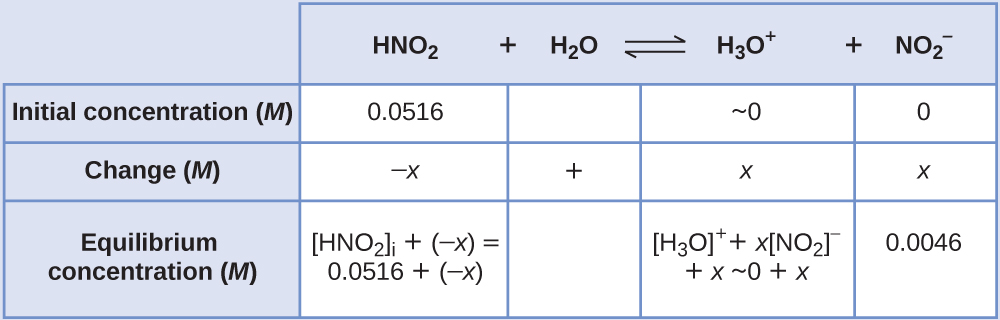
To get the various values in the \(\ce{ICE}\) (Initial, Change, Equilibrium) table, we first calculate [H3O+], the equilibrium concentration of \(\ce{H3O+}\), from the pH:
\([\ce{H3O+}]_{\mathrm{eq}}\) \(= {10}^{(-\mathrm{pH})}\)
\(\ \ \ ={10}^{-2.34}\)
\(\ \ \ =4.6\times 10^{-3}\)
The change in concentration of \(\ce{H3O+}\), \(Δ[\ce{H3O+}]\), is the difference between the equilibrium concentration of \(\ce{H3O+}\), which we determined from the pH, and the initial concentration, [H3O]i. The initial concentration of \(\ce{H3O+}\) is its concentration in pure water, which is so much less than the final concentration that we approximate it as zero (~0).
\(Δ[\ce{H3O+}]\) \(= [\ce{H3O+}]_{\mathrm{eq}} - 0\)
\(\ \ \ =4.6\times 10^{-3} - 0\)
\(\ \ \ =4.6\times 10^{-3}\)
The change in concentration of \(\ce{NO2-}\) is equal to the change in concentration of [H3O+]. For each 1 mol of \(\ce{H3O+}\) that forms, 1 mol of \(\ce{NO2-}\) forms. The equilibrium concentration of \(\ce{HNO2}\) is equal to its initial concentration plus the change in its concentration.
\(Δ[\ce{NO2-}]\) \(= Δ[\ce{H3O+}]\)
\(\ \ \ =4.6\times 10^{-3}\)
\(\ \ \ =4.6\times 10^{-3}\)
\(Δ[\ce{HNO2}]\) \(= -Δ[\ce{H3O+}]\)
\(\ \ \ =-4.6\times 10^{-3}\)
\([\ce{NO2-}]_{\mathrm{eq}}\) \(= Δ[\ce{NO2-}] + 0\)
\(\ \ \ =4.6\times 10^{-3} + 0\)
\(\ \ \ =4.6\times 10^{-3}\)
\([\ce{HNO2}]_{\mathrm{eq}}\) \(= Δ[\ce{HNO2}] + [\ce{HNO2}]_{\mathrm{init}}\)
\(\ \ \ =-4.6\times 10^{-3} + 0.0516\)
\(\ \ \ =0.0470\)
Now we can fill in the \(\ce{ICE}\) table with the concentrations at equilibrium, as shown here:
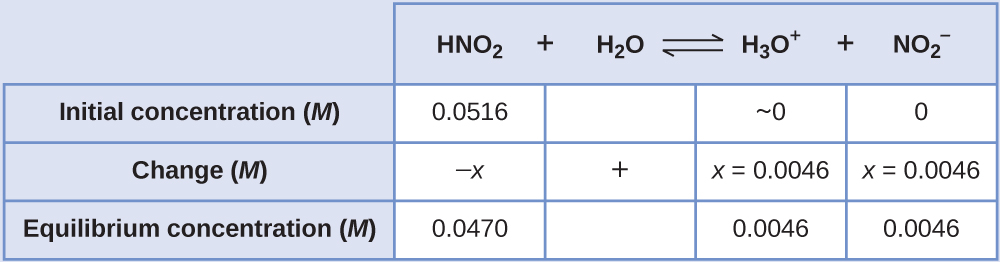
Finally, we calculate the value of the equilibrium constant using the data in the table:
\(K_{\mathrm{a}}\) \(= \dfrac{[\ce{H3O+}]_{\mathrm{eq}} \cdot [\ce{NO2-}]_{\mathrm{eq}}}{[\ce{HNO2}]_{\mathrm{eq}}}\)
\(\ \ \ =\dfrac{4.6\times 10^{-3} \cdot 4.6\times 10^{-3}}{0.0470}\)
\(\ \ \ =\dfrac{2.089\times 10^{-5}}{0.0470}\)
\(\ \ \ =4.4\times 10^{-4}\)