Example 3.23: Calculations using Mass Percentage
“Concentrated” hydrochloric acid is an aqueous solution of 37.2% \(\ce{HCl}\) that is commonly used as a laboratory reagent. The density of this solution is 1.19 g/mL. What mass of \(\ce{HCl}\) is contained in 0.500 L of this solution?Solution
\(f_{\mathrm{HCL\ in\ solution}}\) \(= 37.2\ \mathrm{%}\)\(ρ_{\mathrm{solution}}\) \(= 1.19\ \frac{\mathrm{g}}{\mathrm{mL}}\)
\(V_{\mathrm{solution}}\) \(= 0.500\ \mathrm{L}\)
\(m_{\mathrm{HCl}}\) = ?
The \(\ce{HCl}\) concentration is near 40%, so a 100-g portion of this solution would contain about 40 g of \(\ce{HCl}\). Since the solution density isn’t greatly different from that of water (1 g/mL), a reasonable estimate of the \(\ce{HCl}\) mass in 500 g (0.5 L) of the solution is about five times greater than that in a 100 g portion, or 5 times 40 g equals 200 g.
In order to derive the mass of solute in a solution from its mass percentage, we need to know the corresponding mass of the solution. Using the solution density given, we can convert the solution’s volume to mass, and then use the given mass percentage to calculate the solute mass. This mathematical approach is outlined in this flowchart:
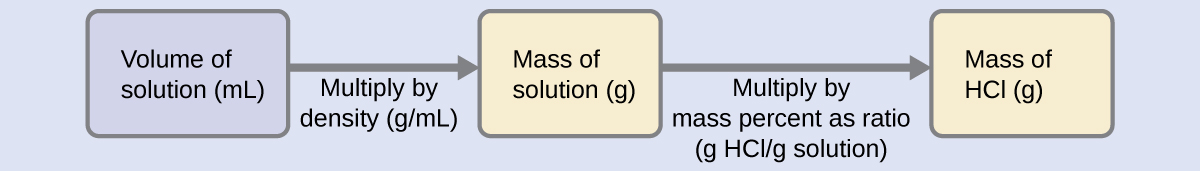
\(m_{\mathrm{solution}}\) \(= ρ_{\mathrm{solution}} \cdot V_{\mathrm{solution}}\)
\(\ \ \ =1.19\ \frac{\mathrm{g}}{\mathrm{mL}} \cdot 0.500\ \mathrm{L}\)
\(\ \ \ =595.\ \mathrm{g}\)
\(m_{\mathrm{HCl}}\) \(= f_{\mathrm{HCL\ in\ solution}} \cdot m_{\mathrm{solution}}\)
\(\ \ \ =37.2\ \mathrm{%} \cdot 595.\ \mathrm{g}\)
\(\ \ \ =221.\ \mathrm{g}\)
This mass of \(\ce{HCl}\) is consistent with our rough estimate of approximately 200 g.