Example 3.8: Deriving the Number of Atoms and Molecules from the Mass of a Compound
A packet of an artificial sweetener contains 40.0 mg of saccharin \(\ce{(C7H5NO3S)}\), which has the structural formula: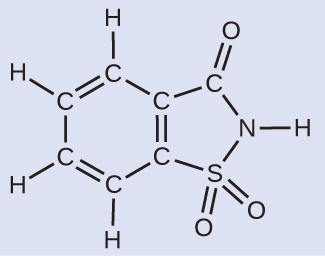
Given that saccharin has a molar mass of 183.18 g/mol, how many saccharin molecules are in a 40.0-mg (0.0400-g) sample of saccharin? How many carbon atoms are in the same sample?
Solution
\(m_{\mathrm{packet}}\) \(= 40.0\ \mathrm{mg}\)\(M_{\mathrm{\ce{C7H5NO3S}}}\) \(= 183.18\ \frac{\mathrm{g}}{\mathrm{mol}}\)
\(N_{\mathrm{molecules}}\) = ?
\(N_{\mathrm{C,atoms}}\) = ?
The number of molecules in a given mass of compound is computed by first deriving the number of moles, as demonstrated in Example 5, and then multiplying by Avogadro’s constant:
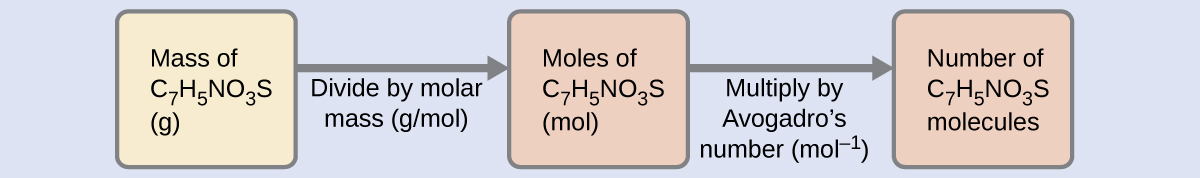
\(N_{\mathrm{molecules}} = \dfrac{m_{\mathrm{sample}}}{M_{\mathrm{compound}}} \cdot N_{\mathrm{A}}\)
\(N_{\mathrm{A}}\) \(= 6.022\times 10^{23}\frac{1}{\mathrm{mol}}\)
Using the provided mass and molar mass for saccharin yields:
\(N_{\mathrm{molecules}}\) \(= \dfrac{m_{\mathrm{packet}}}{M_{\mathrm{\ce{C7H5NNO3S}}}} \cdot N_{\mathrm{A}}\)
\(\ \ \ =\dfrac{40.0\ \mathrm{mg}}{197.19\ \frac{\mathrm{g}}{\mathrm{mol}}} \cdot 6.022\times 10^{23}\frac{1}{\mathrm{mol}}\)
\(\ \ \ =2.0285\times 10^{-4}\ \mathrm{mol} \cdot 6.022\times 10^{23}\frac{1}{\mathrm{mol}}\)
\(\ \ \ =1.222\times 10^{20}\)
The compound’s formula shows that each molecule contains seven carbon atoms, and so the number of C atoms in the provided sample is:
\(N_{\mathrm{C,atoms}}\) \(= 7 \cdot N_{\mathrm{molecules}}\)
\(\ \ \ =7 \cdot 1.222\times 10^{20}\)
\(\ \ \ =8.55\times 10^{20}\)