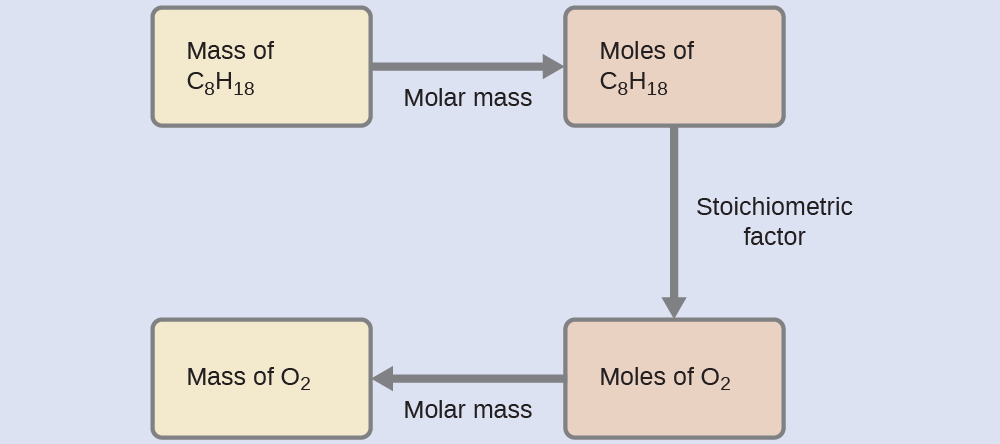
Example 4.11: Relating Masses of Reactants
What mass of oxygen gas, \(\ce{O2}\), from the air is consumed in the combustion of 702 g of octane, \(\ce{C8H18}\), one of the principal components of gasoline?\(\ce{2C8H18}\)\(\ce{ + }\)\(\ce{25O2}\)\(\ce{->}\)\(\ce{16CO2}\)\(\ce{ + }\)\(\ce{18H2O}\)\(\ce{ }\)
Solution
\(m_{\mathrm{\ce{C8H18}}}\) \(= 702.\ \mathrm{g}\)The approach required here is the same as for the Example 9, differing only in that the provided and requested masses are both for reactant species.
\(\dfrac{n_{\mathrm{\ce{O2}}}}{n_{\mathrm{\ce{C8H18}}}} = \frac{25 }{ 2}\)
\(n_{\mathrm{\ce{C8H18}}}\) \(= \dfrac{m_{\mathrm{\ce{C8H18}}}}{M_{\mathrm{\ce{C8H18}}}}\)
\(\ \ \ =\dfrac{702.\ \mathrm{g}}{114.23\ \frac{\mathrm{g}}{\mathrm{mol}}}\)
\(\ \ \ =6.145\ \mathrm{mol}\)
\(n_{\mathrm{\ce{O2}}}\) \(= \frac{25 }{ 2} \cdot n_{\mathrm{\ce{C8H18}}}\)
\(\ \ \ =\frac{25 }{ 2} \cdot 6.145\ \mathrm{mol}\)
\(\ \ \ =76.8\ \mathrm{mol}\)
\(m_{\mathrm{\ce{O2}}}\) \(= n_{\mathrm{\ce{O2}}} \cdot M_{\mathrm{\ce{O2}}}\)
\(\ \ \ =76.8\ \mathrm{mol} \cdot 31.998\ \frac{\mathrm{g}}{\mathrm{mol}}\)
\(\ \ \ =2458.\ \mathrm{g}\)