Example 4.16: Combustion Analysis
Combustion Analysis
Polyethylene is a hydrocarbon polymer used to produce food-storage bags and many other flexible plastic items. A combustion analysis of a 0.00126 g sample of polyethylene yields 0.00394 g of \(\ce{CO2}\) and 0.00161 g of \(\ce{H2O}\). What is the empirical formula of polyethylene?Solution
\(m_{\mathrm{PE}}\) \(= 1.26\times 10^{-3}\ \mathrm{g}\)\(m_{\mathrm{\ce{CO2}}}\) \(= 3.94\times 10^{-3}\ \mathrm{g}\)
\(m_{\mathrm{\ce{H2O}}}\) \(= 1.61\times 10^{-3}\ \mathrm{g}\)
The primary assumption in this exercise is that all the carbon in the sample combusted is converted to carbon dioxide, and all the hydrogen in the sample is converted to water:
\(\ce{CxHy(s)}\)\(\ce{ + }\)\(\ce{excess O2(g)}\)\(\ce{->}\)\(\ce{xCO2(g)}\)\(\ce{ + }\)\(\ce{y/2 H2O(g)}\)\(\ce{ }\)
Note that a balanced equation is not necessary for the task at hand. To derive the empirical formula of the compound, only the subscripts x and y are needed.
First, calculate the chemical amounts of carbon and hydrogen in the sample, using the provided masses of the carbon dioxide and water, respectively. With these chemical amounts, the empirical formula for the compound may be written as described in the previous chapter of this text. An outline of this approach is given in the following flow chart:
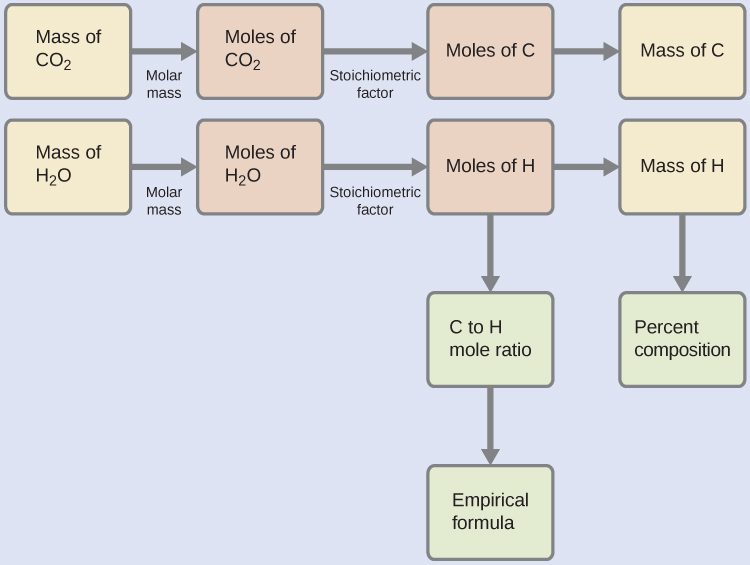
\(n_{\mathrm{\ce{CO2}}}\) \(= \dfrac{m_{\mathrm{\ce{CO2}}}}{M_{\mathrm{\ce{CO2}}}}\)
\(\ \ \ =\dfrac{3.94\times 10^{-3}\ \mathrm{g}}{44.009\ \frac{\mathrm{g}}{\mathrm{mol}}}\)
\(\ \ \ =8.95\times 10^{-5}\ \mathrm{mol}\)
\(n_{\mathrm{\ce{H2O}}}\) \(= \dfrac{m_{\mathrm{\ce{H2O}}}}{M_{\mathrm{\ce{H2O}}}}\)
\(\ \ \ =\dfrac{1.61\times 10^{-3}\ \mathrm{g}}{18.015\ \frac{\mathrm{g}}{\mathrm{mol}}}\)
\(\ \ \ =8.94\times 10^{-5}\ \mathrm{mol}\)
\(n_{\mathrm{\ce{C},inPE}}\) \(= n_{\mathrm{\ce{CO2}}}\)
\(\ \ \ =8.95\times 10^{-5}\ \mathrm{mol}\)
\(\ \ \ =8.95\times 10^{-5}\ \mathrm{mol}\)
\(n_{\mathrm{\ce{H},inPE}}\) \(= n_{\mathrm{\ce{H2O}}} \cdot 2\)
\(\ \ \ =8.94\times 10^{-5}\ \mathrm{mol} \cdot 2\)
\(\ \ \ =1.79\times 10^{-4}\ \mathrm{mol}\)
The empirical formula for the compound is then derived by identifying the smallest whole-number multiples for these molar amounts. The H-to-C molar ratio is
\(\mathrm{ratio}_{\mathrm{HtoC}}\) \(= \dfrac{n_{\mathrm{\ce{H},inPE}}}{n_{\mathrm{\ce{C},inPE}}}\)
\(\ \ \ =\dfrac{1.79\times 10^{-4}\ \mathrm{mol}}{8.95\times 10^{-5}\ \mathrm{mol}}\)
\(\ \ \ =2.00\)
and the empirical formula for polyethylene is \(\ce{CH2}\).