Example 6.10: Quantum Numbers and Electron Configurations
What is the electron configuration and orbital diagram for a phosphorus atom? What are the four quantum numbers for the last electron added?Solution
The atomic number of phosphorus is 15. Thus, a phosphorus atom contains 15 electrons. The order of filling of the energy levels is 1s, 2s, 2p, 3s, 3p, 4s, . . . The 15 electrons of the phosphorus atom will fill up to the 3p orbital, which will contain three electrons:\(\ce{1s^2 2s^2 2p^6 3s^2 3p^3}\)
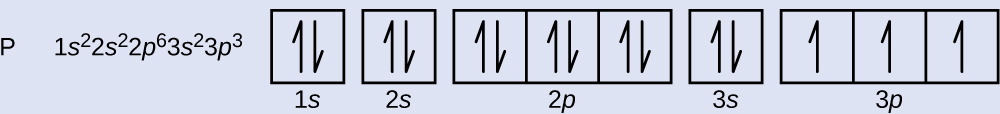
The last electron added is a 3p electron. \(Failed to interpret math QQQTherefore, n QQQ = 3\) and, for a p-type \(Failed to interpret math QQQorbital, l QQQ = 1.\) The \(m_{\mathrm{l}}\) value could be -1, 0, or +1. The three p orbitals are degenerate, so any of these \(m_{\mathrm{l}}\) values is correct. For unpaired electrons, convention assigns the value of +1/2 for the spin quantum number; thus, \(m_{\mathrm{s}}\) is +1/2.