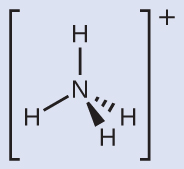
Example 7.12: Predicting Electron-pair Geometry and Molecular Structure: Ammonium
Two of the top 50 chemicals produced in the United States, ammonium nitrate and ammonium sulfate, both used as fertilizers, contain the ammonium ion. Predict the electron-pair geometry and molecular structure of the \(\ce{NH4+}\) cation.Solution
We write the Lewis structure of \(\ce{NH4+}\) as: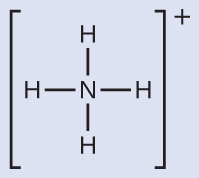
We can see that \(\ce{NH4+}\) contains four bonds from the nitrogen atom to hydrogen atoms and no lone pairs. We expect the four regions of high electron density to arrange themselves so that they point to the corners of a tetrahedron with the central nitrogen atom in the middle ( Figure 19). Therefore, the electron pair geometry of \(\ce{NH4+}\) is tetrahedral, and the molecular structure is also tetrahedral ( Figure 22).