Example 7.16: Predicting Structure in Multicenter Molecules
The Lewis structure for the simplest amino acid, glycine, \(\ce{H2NCH2CO2H}\), is shown here. Predict the local geometry for the nitrogen atom, the two carbon atoms, and the oxygen atom with a hydrogen atom attached: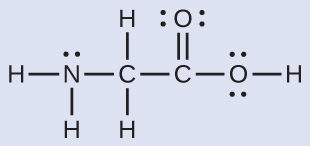
Solution
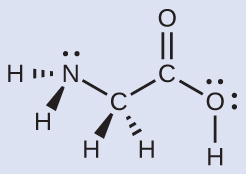
Consider each central atom independently. The electron-pair geometries:
nitrogen––four regions of electron density; tetrahedral
carbon (CH2)––four regions of electron density; tetrahedral
carbon (CO2)—three regions of electron density; trigonal planar
oxygen (OH)—four regions of electron density; tetrahedral
The local structures:
nitrogen––three bonds, one lone pair; trigonal pyramidal
carbon (CH2)—four bonds, no lone pairs; tetrahedral
carbon (CO2)—three bonds (double bond counts as one bond), no lone pairs; trigonal planar
oxygen (OH)—two bonds, two lone pairs; bent (109°)