Example 7.5: Writing Lewis Structures: Octet Rule Violations
Xenon is a noble gas, but it forms a number of stable compounds. We examined \(\ce{XeF4}\) earlier. What are the Lewis structures of \(\ce{XeF2}\) and \(\ce{XeF6}\)?Solution
We can draw the Lewis structure of any covalent molecule by following the six steps discussed earlier. In this case, we can condense the last few steps, since not all of them apply.Calculate the number of valence electrons:
\(\mathrm{VE}_{\mathrm{\ce{XeF2}}}\) \(= 8 + 2 \cdot 7\)
\(\ \ \ =8 + 14\)
\(\ \ \ =22\)
\(\mathrm{VE}_{\mathrm{\ce{XeF6}}}\) \(= 8 + 6 \cdot 7\)
\(\ \ \ =8 + 42\)
\(\ \ \ =50\)
Draw a skeleton joining the atoms by single bonds. Xenon will be the central atom because fluorine cannot be a central atom:
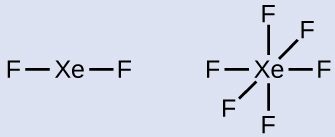
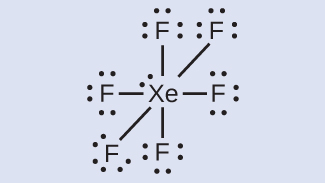
Distribute the remaining electrons.
\(\ce{XeF2:}\) We place three lone pairs of electrons around each F atom, accounting for 12 electrons and giving each F atom 8 electrons. Thus, six electrons (three lone pairs) remain. These lone pairs must be placed on the Xe atom. This is acceptable because Xe atoms have empty valence shell d orbitals and can accommodate more than eight electrons. The Lewis structure of \(\ce{XeF2}\) shows two bonding pairs and three lone pairs of electrons around the Xe atom:
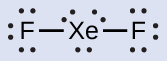
\(\ce{XeF6:}\) We place three lone pairs of electrons around each F atom, accounting for 36 electrons. Two electrons remain, and this lone pair is placed on the Xe atom: