Example 7.7: Calculating Formal Charge from Lewis Structures
Assign formal charges to each atom in the interhalogen molecule \(\ce{BrCl3}\).Solution
Assign one of the electrons in each Br–Cl bond to the Br atom and one to the Cl atom in that bond: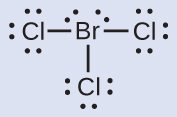
Assign the lone pairs to their atom. Now each Cl atom has seven electrons and the Br atom has seven electrons.
Subtract this number from the number of valence electrons for the neutral atom. This gives the formal charge:
\(\mathrm{FC}_{\mathrm{\ce{Br}}}\) \(= 7 - 7\)
\(\ \ \ =0\)
\(\mathrm{FC}_{\mathrm{\ce{Cl}}}\) \(= 7 - 7\)
\(\ \ \ =0\)
All atoms in \(\ce{BrCl3}\) have a formal charge of zero, and the sum of the formal charges totals zero, as it must in a neutral molecule.