Example 7.9: Using Bond Energies to Calculate Approximate Enthalpy Changes
Methanol, \(\ce{CH3OH}\), may be an excellent alternative fuel. The high-temperature reaction of steam and carbon produces a mixture of the gases carbon monoxide, \(\ce{CO}\), and hydrogen, \(\ce{H2}\), from which methanol can be produced. Using the bond energies in Table 4, calculate the approximate enthalpy change, \(ΔH\), for the reaction here:\(\ce{CO(g)}\)\(\ce{ + }\)\(\ce{2H2(g)}\)\(\ce{->}\)\(\ce{CH3OH(g)}\)\(\ce{ }\)
Solution
First, we need to write the Lewis structures of the reactants and the products: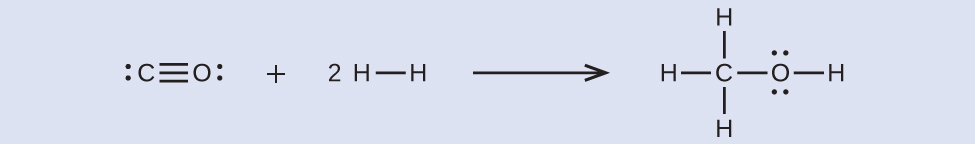
From this, we see that \(ΔH\) for this reaction involves the energy required to break a C–O triple bond and two H–H single bonds, as well as the energy produced by the formation of three C–H single bonds, a C–O single bond, and an O–H single bond. We can express this as follows:
\(ΔH = \sum (D_{\mathrm{bonds\ broken}}) - \sum (D_{\mathrm{bonds\ formed}})\)
\(ΔH = \sum (D_{\mathrm{\ce{C#O}}}, 2 \cdot D_{\mathrm{\ce{H-H}}}) - \sum (3 \cdot D_{\mathrm{\ce{C-H}}}, D_{\mathrm{\ce{C-O}}}, D_{\mathrm{\ce{O-H}}})\)
Using the bond energy values in Table 4, we obtain:
\(D_{\mathrm{\ce{C#O}}}\) \(= 1080\ \frac{\mathrm{kJ}}{\mathrm{mol}}\)
\(D_{\mathrm{\ce{H-H}}}\) \(= 436\ \frac{\mathrm{kJ}}{\mathrm{mol}}\)
\(D_{\mathrm{\ce{C-H}}}\) \(= 415\ \frac{\mathrm{kJ}}{\mathrm{mol}}\)
\(D_{\mathrm{\ce{C-O}}}\) \(= 350\ \frac{\mathrm{kJ}}{\mathrm{mol}}\)
\(D_{\mathrm{\ce{O-H}}}\) \(= 464\ \frac{\mathrm{kJ}}{\mathrm{mol}}\)
\(ΔH_{\mathrm{estimate}}\) \(= \sum (D_{\mathrm{\ce{C#O}}}, 2 \cdot D_{\mathrm{\ce{H-H}}}) - \sum (3 \cdot D_{\mathrm{\ce{C-H}}}, D_{\mathrm{\ce{C-O}}}, D_{\mathrm{\ce{O-H}}})\)
\(\ \ \ =\sum (1080\ \frac{\mathrm{kJ}}{\mathrm{mol}}, 2 \cdot 436\ \frac{\mathrm{kJ}}{\mathrm{mol}}) - \sum (3 \cdot 415\ \frac{\mathrm{kJ}}{\mathrm{mol}}, 350\ \frac{\mathrm{kJ}}{\mathrm{mol}}, 464\ \frac{\mathrm{kJ}}{\mathrm{mol}})\)
\(\ \ \ =\sum (1080\ \frac{\mathrm{kJ}}{\mathrm{mol}}, 872\ \frac{\mathrm{kJ}}{\mathrm{mol}}) - \sum (1245\ \frac{\mathrm{kJ}}{\mathrm{mol}}, 350\ \frac{\mathrm{kJ}}{\mathrm{mol}}, 464\ \frac{\mathrm{kJ}}{\mathrm{mol}})\)
\(\ \ \ =1952\ \frac{\mathrm{kJ}}{\mathrm{mol}} - 2059\ \frac{\mathrm{kJ}}{\mathrm{mol}}\)
\(\ \ \ =-107\ \frac{\mathrm{kJ}}{\mathrm{mol}}\)
We can compare this value to the value calculated based on \(\mathrm{ΔHf°}\) data from Appendix G:
\(\mathrm{ΔHf°}_{\mathrm{\ce{CH3OH(g)}}}\) \(= -201.0\ \frac{\mathrm{kJ}}{\mathrm{mol}}\)
\(\mathrm{ΔHf°}_{\mathrm{\ce{CO(g)}}}\) \(= -110.52\ \frac{\mathrm{kJ}}{\mathrm{mol}}\)
\(\mathrm{ΔHf°}_{\mathrm{\ce{H2}}}\) \(= 0\)
\(ΔH_{\mathrm{measurement}}\) \(= \mathrm{ΔHf°}_{\mathrm{\ce{CH3OH(g)}}} - \mathrm{ΔHf°}_{\mathrm{\ce{CO(g)}}} - 2 \cdot \mathrm{ΔHf°}_{\mathrm{\ce{H2}}}\)
\(\ \ \ =-201.0\ \frac{\mathrm{kJ}}{\mathrm{mol}} - (-110.52\ \frac{\mathrm{kJ}}{\mathrm{mol}}) - 2 \cdot 0\)
\(\ \ \ =-90.48\ \frac{\mathrm{kJ}}{\mathrm{mol}} - 0\)
\(\ \ \ =-90.5\ \frac{\mathrm{kJ}}{\mathrm{mol}}\)
Note that there is a fairly significant gap between the values calculated using the two different methods. This occurs because D values are the average of different bond strengths; therefore, they often give only rough agreement with other data.