This content is available for free here
Chapter 1: Essential Ideas
Chapter 1.1: Chemistry in Context
Chemistry deals with the composition, structure, and properties of matter, and the ways by which various forms of matter may be interconverted. Thus, it occupies a central place in the study and practice of science and technology. Chemists use the scientific method to perform experiments, pose hypotheses, and formulate laws and develop theories, so that they can better understand the behavior of the natural world. To do so, they operate in the macroscopic, microscopic, and symbolic domains. Chemists measure, analyze, purify, and synthesize a wide variety of substances that are important to our lives.
Chapter 1.2: Phases and Classification of Matter
Matter is anything that occupies space and has mass. The basic building block of matter is the atom, the smallest unit of an element that can enter into combinations with atoms of the same or other elements. In many substances, atoms are combined into molecules. On earth, matter commonly exists in three states: solids, of fixed shape and volume; liquids, of variable shape but fixed volume; and gases, of variable shape and volume. Under high-temperature conditions, matter also can exist as a plasma. Most matter is a mixture: It is composed of two or more types of matter that can be present in varying amounts and can be separated by physical means. Heterogeneous mixtures vary in composition from point to point; homogeneous mixtures have the same composition from point to point. Pure substances consist of only one type of matter. A pure substance can be an element, which consists of only one type of atom and cannot be broken down by a chemical change, or a compound, which consists of two or more types of atoms.
Chapter 1.3: Physical and Chemical Properties
All substances have distinct physical and chemical properties, and may undergo physical or chemical changes. Physical properties, such as hardness and boiling point, and physical changes, such as melting or freezing, do not involve a change in the composition of matter. Chemical properties, such flammability and acidity, and chemical changes, such as rusting, involve production of matter that differs from that present beforehand.
Measurable properties fall into one of two categories. Extensive properties depend on the amount of matter present, for example, the mass of gold. Intensive properties do not depend on the amount of matter present, for example, the density of gold. Heat is an example of an extensive property, and temperature is an example of an intensive property.
Chapter 1.4: Measurements
Measurements provide quantitative information that is critical in studying and practicing chemistry. Each measurement has an amount, a unit for comparison, and an uncertainty. Measurements can be represented in either decimal or scientific notation. Scientists primarily use the SI (International System) or metric systems. We use base SI units such as meters, seconds, and kilograms, as well as derived units, such as liters (for volume) and g/cm3 (for density). In many cases, we find it convenient to use unit prefixes that yield fractional and multiple units, such as microseconds (10−6 seconds) and megahertz (106 hertz), respectively.
Chapter 1.5: Measurement Uncertainty, Accuracy, and Precision
Quantities can be exact or measured. Measured quantities have an associated uncertainty that is represented by the number of significant figures in the measurement. The uncertainty of a calculated value depends on the uncertainties in the values used in the calculation and is reflected in how the value is rounded. Measured values can be accurate (close to the true value) and/or precise (showing little variation when measured repeatedly).
Chapter 1.6: Mathematical Treatment of Measurement Results
Measurements are made using a variety of units. It is often useful or necessary to convert a measured quantity from one unit into another. These conversions are accomplished using unit conversion factors, which are derived by simple applications of a mathematical approach called the factor-label method or dimensional analysis. This strategy is also employed to calculate sought quantities using measured quantities and appropriate mathematical relations.
This content is available for free here
Chapter 2: Atoms, Molecules, and Ions
Chapter 2.1: Early Ideas in Atomic Theory
The ancient Greeks proposed that matter consists of extremely small particles called atoms. Dalton postulated that each element has a characteristic type of atom that differs in properties from atoms of all other elements, and that atoms of different elements can combine in fixed, small, whole-number ratios to form compounds. Samples of a particular compound all have the same elemental proportions by mass. When two elements form different compounds, a given mass of one element will combine with masses of the other element in a small, whole-number ratio. During any chemical change, atoms are neither created nor destroyed.
Chapter 2.2: Evolution of Atomic Theory
Although no one has actually seen the inside of an atom, experiments have demonstrated much about atomic structure. Thomson’s cathode ray tube showed that atoms contain small, negatively charged particles called electrons. Millikan discovered that there is a fundamental electric charge—the charge of an electron. Rutherford’s gold foil experiment showed that atoms have a small, dense, positively charged nucleus; the positively charged particles within the nucleus are called protons. Chadwick discovered that the nucleus also contains neutral particles called neutrons. Soddy demonstrated that atoms of the same element can differ in mass; these are called isotopes.
Chapter 2.3: Atomic Structure and Symbolism
An atom consists of a small, positively charged nucleus surrounded by electrons. The nucleus contains protons and neutrons; its diameter is about 100,000 times smaller than that of the atom. The mass of one atom is usually expressed in atomic mass units (amu), which is referred to as the atomic mass. An amu is defined as exactly of the mass of a carbon-12 atom and is equal to 1.6605 10−24 g.
Protons are relatively heavy particles with a charge of 1+ and a mass of 1.0073 amu. Neutrons are relatively heavy particles with no charge and a mass of 1.0087 amu. Electrons are light particles with a charge of 1− and a mass of 0.00055 amu. The number of protons in the nucleus is called the atomic number (Z) and is the property that defines an atom’s elemental identity. The sum of the numbers of protons and neutrons in the nucleus is called the mass number and, expressed in amu, is approximately equal to the mass of the atom. An atom is neutral when it contains equal numbers of electrons and protons.
Isotopes of an element are atoms with the same atomic number but different mass numbers; isotopes of an element, therefore, differ from each other only in the number of neutrons within the nucleus. When a naturally occurring element is composed of several isotopes, the atomic mass of the element represents the average of the masses of the isotopes involved. A chemical symbol identifies the atoms in a substance using symbols, which are one-, two-, or three-letter abbreviations for the atoms.
Chapter 2.4: Chemical Formulas
A molecular formula uses chemical symbols and subscripts to indicate the exact numbers of different atoms in a molecule or compound. An empirical formula gives the simplest, whole-number ratio of atoms in a compound. A structural formula indicates the bonding arrangement of the atoms in the molecule. Ball-and-stick and space-filling models show the geometric arrangement of atoms in a molecule. Isomers are compounds with the same molecular formula but different arrangements of atoms.
Chapter 2.5: The Periodic Table
The discovery of the periodic recurrence of similar properties among the elements led to the formulation of the periodic table, in which the elements are arranged in order of increasing atomic number in rows known as periods and columns known as groups. Elements in the same group of the periodic table have similar chemical properties. Elements can be classified as metals, metalloids, and nonmetals, or as a main-group elements, transition metals, and inner transition metals. Groups are numbered 1–18 from left to right. The elements in group 1 are known as the alkali metals; those in group 2 are the alkaline earth metals; those in 15 are the pnictogens; those in 16 are the chalcogens; those in 17 are the halogens; and those in 18 are the noble gases.
Chapter 2.6: Molecular and Ionic Compounds
Metals (particularly those in groups 1 and 2) tend to lose the number of electrons that would leave them with the same number of electrons as in the preceding noble gas in the periodic table. By this means, a positively charged ion is formed. Similarly, nonmetals (especially those in groups 16 and 17, and, to a lesser extent, those in Group 15) can gain the number of electrons needed to provide atoms with the same number of electrons as in the next noble gas in the periodic table. Thus, nonmetals tend to form negative ions. Positively charged ions are called cations, and negatively charged ions are called anions. Ions can be either monatomic (containing only one atom) or polyatomic (containing more than one atom).
Compounds that contain ions are called ionic compounds. Ionic compounds generally form from metals and nonmetals. Compounds that do not contain ions, but instead consist of atoms bonded tightly together in molecules (uncharged groups of atoms that behave as a single unit), are called covalent compounds. Covalent compounds usually form from two nonmetals.
Chapter 2.7: Chemical Nomenclature
Chemists use nomenclature rules to clearly name compounds. Ionic and molecular compounds are named using somewhat-different methods. Binary ionic compounds typically consist of a metal and a nonmetal. The name of the metal is written first, followed by the name of the nonmetal with its ending changed to –ide. For example, K2O is called potassium oxide. If the metal can form ions with different charges, a Roman numeral in parentheses follows the name of the metal to specify its charge. Thus, FeCl2 is iron(II) chloride and FeCl3 is iron(III) chloride. Some compounds contain polyatomic ions; the names of common polyatomic ions should be memorized. Molecular compounds can form compounds with different ratios of their elements, so prefixes are used to specify the numbers of atoms of each element in a molecule of the compound. Examples include SF6, sulfur hexafluoride, and N2O4, dinitrogen tetroxide. Acids are an important class of compounds containing hydrogen and having special nomenclature rules. Binary acids are named using the prefix hydro-, changing the –ide suffix to –ic, and adding “acid;” HCl is hydrochloric acid. Oxyacids are named by changing the ending of the anion to –ic, and adding “acid;” H2CO3 is carbonic acid.
This content is available for free here
Chapter 3: Composition of Substances and Solutions
Chapter 3.1: Formula Mass and the Mole Concept
The formula mass of a substance is the sum of the average atomic masses of each atom represented in the chemical formula and is expressed in atomic mass units. The formula mass of a covalent compound is also called the molecular mass. A convenient amount unit for expressing very large numbers of atoms or molecules is the mole. Experimental measurements have determined the number of entities composing 1 mole of substance to be 6.022 1023, a quantity called Avogadro’s number. The mass in grams of 1 mole of substance is its molar mass. Due to the use of the same reference substance in defining the atomic mass unit and the mole, the formula mass (amu) and molar mass (g/mol) for any substance are numerically equivalent (for example, one H2O molecule weighs approximately18 amu and 1 mole of H2O molecules weighs approximately 18 g).
Chapter 3.2: Determining Empirical and Molecular Formulas
The chemical identity of a substance is defined by the types and relative numbers of atoms composing its fundamental entities (molecules in the case of covalent compounds, ions in the case of ionic compounds). A compound’s percent composition provides the mass percentage of each element in the compound, and it is often experimentally determined and used to derive the compound’s empirical formula. The empirical formula mass of a covalent compound may be compared to the compound’s molecular or molar mass to derive a molecular formula.
Chapter 3.3: Molarity
Solutions are homogeneous mixtures. Many solutions contain one component, called the solvent, in which other components, called solutes, are dissolved. An aqueous solution is one for which the solvent is water. The concentration of a solution is a measure of the relative amount of solute in a given amount of solution. Concentrations may be measured using various units, with one very useful unit being molarity, defined as the number of moles of solute per liter of solution. The solute concentration of a solution may be decreased by adding solvent, a process referred to as dilution. The dilution equation is a simple relation between concentrations and volumes of a solution before and after dilution.

Chapter 3.4: Other Units for Solution Concentrations
In addition to molarity, a number of other solution concentration units are used in various applications. Percentage concentrations based on the solution components’ masses, volumes, or both are useful for expressing relatively high concentrations, whereas lower concentrations are conveniently expressed using ppm or ppb units. These units are popular in environmental, medical, and other fields where mole-based units such as molarity are not as commonly used.
This content is available for free here
Chapter 4: Stoichiometry of Chemical Reactions
Chapter 4.1: Writing and Balancing Chemical Equations
Chemical equations are symbolic representations of chemical and physical changes. Formulas for the substances undergoing the change (reactants) and substances generated by the change (products) are separated by an arrow and preceded by integer coefficients indicating their relative numbers. Balanced equations are those whose coefficients result in equal numbers of atoms for each element in the reactants and products. Chemical reactions in aqueous solution that involve ionic reactants or products may be represented more realistically by complete ionic equations and, more succinctly, by net ionic equations.
Chapter 4.2: Classifying Chemical Reactions
Chemical reactions are classified according to similar patterns of behavior. A large number of important reactions are included in three categories: precipitation, acid-base, and oxidation-reduction (redox). Precipitation reactions involve the formation of one or more insoluble products. Acid-base reactions involve the transfer of hydrogen ions between reactants. Redox reactions involve a change in oxidation number for one or more reactant elements. Writing balanced equations for some redox reactions that occur in aqueous solutions is simplified by using a systematic approach called the half-reaction method.
Chapter 4.3: Reaction Stoichiometry
A balanced chemical equation may be used to describe a reaction’s stoichiometry (the relationships between amounts of reactants and products). Coefficients from the equation are used to derive stoichiometric factors that subsequently may be used for computations relating reactant and product masses, molar amounts, and other quantitative properties.
Chapter 4.4: Reaction Yields
When reactions are carried out using less-than-stoichiometric quantities of reactants, the amount of product generated will be determined by the limiting reactant. The amount of product generated by a chemical reaction is its actual yield. This yield is often less than the amount of product predicted by the stoichiometry of the balanced chemical equation representing the reaction (its theoretical yield). The extent to which a reaction generates the theoretical amount of product is expressed as its percent yield.
Chapter 4.5: Quantitative Chemical Analysis
The stoichiometry of chemical reactions may serve as the basis for quantitative chemical analysis methods. Titrations involve measuring the volume of a titrant solution required to completely react with a sample solution. This volume is then used to calculate the concentration of analyte in the sample using the stoichiometry of the titration reaction. Gravimetric analysis involves separating the analyte from the sample by a physical or chemical process, determining its mass, and then calculating its concentration in the sample based on the stoichiometry of the relevant process. Combustion analysis is a gravimetric method used to determine the elemental composition of a compound by collecting and weighing the gaseous products of its combustion.
This content is available for free here
Chapter 5: Thermochemistry
Chapter 5.1: Energy Basics
Energy is the capacity to do work (applying a force to move matter). Kinetic energy (KE) is the energy of motion; potential energy is energy due to relative position, composition, or condition. When energy is converted from one form into another, energy is neither created nor destroyed (law of conservation of energy or first law of thermodynamics).
Matter has thermal energy due to the KE of its molecules and temperature that corresponds to the average KE of its molecules. Heat is energy that is transferred between objects at different temperatures; it flows from a high to a low temperature. Chemical and physical processes can absorb heat (endothermic) or release heat (exothermic). The SI unit of energy, heat, and work is the joule (J).
Specific heat and heat capacity are measures of the energy needed to change the temperature of a substance or object. The amount of heat absorbed or released by a substance depends directly on the type of substance, its mass, and the temperature change it undergoes.
Chapter 5.2: Calorimetry
Calorimetry is used to measure the amount of thermal energy transferred in a chemical or physical process. This requires careful measurement of the temperature change that occurs during the process and the masses of the system and surroundings. These measured quantities are then used to compute the amount of heat produced or consumed in the process using known mathematical relations.
Calorimeters are designed to minimize energy exchange between the system being studied and its surroundings. They range from simple coffee cup calorimeters used by introductory chemistry students to sophisticated bomb calorimeters used to determine the energy content of food.
Chapter 5.3: Enthalpy
If a chemical change is carried out at constant pressure and the only work done is caused by expansion or contraction, q for the change is called the enthalpy change with the symbol ΔH, or for reactions occurring under standard state conditions. The value of ΔH for a reaction in one direction is equal in magnitude, but opposite in sign, to ΔH for the reaction in the opposite direction, and ΔH is directly proportional to the quantity of reactants and products. Examples of enthalpy changes include enthalpy of combustion, enthalpy of fusion, enthalpy of vaporization, and standard enthalpy of formation. The standard enthalpy of formation, is the enthalpy change accompanying the formation of 1 mole of a substance from the elements in their most stable states at 1 bar (standard state). Many of the processes are carried out at 298.15 K. If the enthalpies of formation are available for the reactants and products of a reaction, the enthalpy change can be calculated using Hess’s law: If a process can be written as the sum of several stepwise processes, the enthalpy change of the total process equals the sum of the enthalpy changes of the various steps.
This content is available for free here
Chapter 6: Electronic Structures and Periodic Properties of Elements
Chapter 6.1: Electromagnetic Energy
Light and other forms of electromagnetic radiation move through a vacuum with a constant speed, c, of 2.998 108 m s−1. This radiation shows wavelike behavior, which can be characterized by a frequency, ν, and a wavelength, λ, such that c = λν. Light is an example of a travelling wave. Other important wave phenomena include standing waves, periodic oscillations, and vibrations. Standing waves exhibit quantization, since their wavelengths are limited to discrete integer multiples of some characteristic lengths. Electromagnetic radiation that passes through two closely spaced narrow slits having dimensions roughly similar to the wavelength will show an interference pattern that is a result of constructive and destructive interference of the waves. Electromagnetic radiation also demonstrates properties of particles called photons. The energy of a photon is related to the frequency (or alternatively, the wavelength) of the radiation as E = hν (or ), where h is Planck's constant. That light demonstrates both wavelike and particle-like behavior is known as wave-particle duality. All forms of electromagnetic radiation share these properties, although various forms including X-rays, visible light, microwaves, and radio waves interact differently with matter and have very different practical applications. Electromagnetic radiation can be generated by exciting matter to higher energies, such as by heating it. The emitted light can be either continuous (incandescent sources like the sun) or discrete (from specific types of excited atoms). Continuous spectra often have distributions that can be approximated as blackbody radiation at some appropriate temperature. The line spectrum of hydrogen can be obtained by passing the light from an electrified tube of hydrogen gas through a prism. This line spectrum was simple enough that an empirical formula called the Rydberg formula could be derived from the spectrum. Three historically important paradoxes from the late 19th and early 20th centuries that could not be explained within the existing framework of classical mechanics and classical electromagnetism were the blackbody problem, the photoelectric effect, and the discrete spectra of atoms. The resolution of these paradoxes ultimately led to quantum theories that superseded the classical theories.
Chapter 6.2: The Bohr Model
Bohr incorporated Planck’s and Einstein’s quantization ideas into a model of the hydrogen atom that resolved the paradox of atom stability and discrete spectra. The Bohr model of the hydrogen atom explains the connection between the quantization of photons and the quantized emission from atoms. Bohr described the hydrogen atom in terms of an electron moving in a circular orbit about a nucleus. He postulated that the electron was restricted to certain orbits characterized by discrete energies. Transitions between these allowed orbits result in the absorption or emission of photons. When an electron moves from a higher-energy orbit to a more stable one, energy is emitted in the form of a photon. To move an electron from a stable orbit to a more excited one, a photon of energy must be absorbed. Using the Bohr model, we can calculate the energy of an electron and the radius of its orbit in any one-electron system.
Chapter 6.3: Development of Quantum Theory
Macroscopic objects act as particles. Microscopic objects (such as electrons) have properties of both a particle and a wave. Their exact trajectories cannot be determined. The quantum mechanical model of atoms describes the three-dimensional position of the electron in a probabilistic manner according to a mathematical function called a wavefunction, often denoted as ψ. Atomic wavefunctions are also called orbitals. The squared magnitude of the wavefunction describes the distribution of the probability of finding the electron in a particular region in space. Therefore, atomic orbitals describe the areas in an atom where electrons are most likely to be found.
An atomic orbital is characterized by three quantum numbers. The principal quantum number, n, can be any positive integer. The general region for value of energy of the orbital and the average distance of an electron from the nucleus are related to n. Orbitals having the same value of n are said to be in the same shell. The angular momentum quantum number, l, can have any integer value from 0 to n – 1. This quantum number describes the shape or type of the orbital. Orbitals with the same principle quantum number and the same l value belong to the same subshell. The magnetic quantum number, ml, with 2l + 1 values ranging from –l to +l, describes the orientation of the orbital in space. In addition, each electron has a spin quantum number, ms, that can be equal to No two electrons in the same atom can have the same set of values for all the four quantum numbers.
Chapter 6.4: Electronic Structure of Atoms (Electron Configurations)
The relative energy of the subshells determine the order in which atomic orbitals are filled (1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, and so on). Electron configurations and orbital diagrams can be determined by applying the Pauli exclusion principle (no two electrons can have the same set of four quantum numbers) and Hund’s rule (whenever possible, electrons retain unpaired spins in degenerate orbitals).
Electrons in the outermost orbitals, called valence electrons, are responsible for most of the chemical behavior of elements. In the periodic table, elements with analogous valence electron configurations usually occur within the same group. There are some exceptions to the predicted filling order, particularly when half-filled or completely filled orbitals can be formed. The periodic table can be divided into three categories based on the orbital in which the last electron to be added is placed: main group elements (s and p orbitals), transition elements (d orbitals), and inner transition elements (f orbitals).
Chapter 6.5: Periodic Variations in Element Properties
Electron configurations allow us to understand many periodic trends. Covalent radius increases as we move down a group because the n level (orbital size) increases. Covalent radius mostly decreases as we move left to right across a period because the effective nuclear charge experienced by the electrons increases, and the electrons are pulled in tighter to the nucleus. Anionic radii are larger than the parent atom, while cationic radii are smaller, because the number of valence electrons has changed while the nuclear charge has remained constant. Ionization energy (the energy associated with forming a cation) decreases down a group and mostly increases across a period because it is easier to remove an electron from a larger, higher energy orbital. Electron affinity (the energy associated with forming an anion) is more favorable (exothermic) when electrons are placed into lower energy orbitals, closer to the nucleus. Therefore, electron affinity becomes increasingly negative as we move left to right across the periodic table and decreases as we move down a group. For both IE and electron affinity data, there are exceptions to the trends when dealing with completely filled or half-filled subshells.
This content is available for free here
Chapter 7: Chemical Bonding and Molecular Geometry
Chapter 7.1: Ionic Bonding
Atoms gain or lose electrons to form ions with particularly stable electron configurations. The charges of cations formed by the representative metals may be determined readily because, with few exceptions, the electronic structures of these ions have either a noble gas configuration or a completely filled electron shell. The charges of anions formed by the nonmetals may also be readily determined because these ions form when nonmetal atoms gain enough electrons to fill their valence shells.
Chapter 7.2: Covalent Bonding
Covalent bonds form when electrons are shared between atoms and are attracted by the nuclei of both atoms. In pure covalent bonds, the electrons are shared equally. In polar covalent bonds, the electrons are shared unequally, as one atom exerts a stronger force of attraction on the electrons than the other. The ability of an atom to attract a pair of electrons in a chemical bond is called its electronegativity. The difference in electronegativity between two atoms determines how polar a bond will be. In a diatomic molecule with two identical atoms, there is no difference in electronegativity, so the bond is nonpolar or pure covalent. When the electronegativity difference is very large, as is the case between metals and nonmetals, the bonding is characterized as ionic.
Chapter 7.3: Lewis Symbols and Structures
Valence electronic structures can be visualized by drawing Lewis symbols (for atoms and monatomic ions) and Lewis structures (for molecules and polyatomic ions). Lone pairs, unpaired electrons, and single, double, or triple bonds are used to indicate where the valence electrons are located around each atom in a Lewis structure. Most structures—especially those containing second row elements—obey the octet rule, in which every atom (except H) is surrounded by eight electrons. Exceptions to the octet rule occur for odd-electron molecules (free radicals), electron-deficient molecules, and hypervalent molecules.
Chapter 7.4: Formal Charges and Resonance
In a Lewis structure, formal charges can be assigned to each atom by treating each bond as if one-half of the electrons are assigned to each atom. These hypothetical formal charges are a guide to determining the most appropriate Lewis structure. A structure in which the formal charges are as close to zero as possible is preferred. Resonance occurs in cases where two or more Lewis structures with identical arrangements of atoms but different distributions of electrons can be written. The actual distribution of electrons (the resonance hybrid) is an average of the distribution indicated by the individual Lewis structures (the resonance forms).
Chapter 7.5: Strengths of Ionic and Covalent Bonds
The strength of a covalent bond is measured by its bond dissociation energy, that is, the amount of energy required to break that particular bond in a mole of molecules. Multiple bonds are stronger than single bonds between the same atoms. The enthalpy of a reaction can be estimated based on the energy input required to break bonds and the energy released when new bonds are formed. For ionic bonds, the lattice energy is the energy required to separate one mole of a compound into its gas phase ions. Lattice energy increases for ions with higher charges and shorter distances between ions. Lattice energies are often calculated using the Born-Haber cycle, a thermochemical cycle including all of the energetic steps involved in converting elements into an ionic compound.
Chapter 7.6: Molecular Structure and Polarity
VSEPR theory predicts the three-dimensional arrangement of atoms in a molecule. It states that valence electrons will assume an electron-pair geometry that minimizes repulsions between areas of high electron density (bonds and/or lone pairs). Molecular structure, which refers only to the placement of atoms in a molecule and not the electrons, is equivalent to electron-pair geometry only when there are no lone electron pairs around the central atom. A dipole moment measures a separation of charge. For one bond, the bond dipole moment is determined by the difference in electronegativity between the two atoms. For a molecule, the overall dipole moment is determined by both the individual bond moments and how these dipoles are arranged in the molecular structure. Polar molecules (those with an appreciable dipole moment) interact with electric fields, whereas nonpolar molecules do not.
This content is available for free here
Chapter 8: Advanced Theories of Covalent Bonding
Chapter 8.1: Valence Bond Theory
Valence bond theory describes bonding as a consequence of the overlap of two separate atomic orbitals on different atoms that creates a region with one pair of electrons shared between the two atoms. When the orbitals overlap along an axis containing the nuclei, they form a σ bond. When they overlap in a fashion that creates a node along this axis, they form a π bond.
Chapter 8.2: Hybrid Atomic Orbitals
We can use hybrid orbitals, which are mathematical combinations of some or all of the valence atomic orbitals, to describe the electron density around covalently bonded atoms. These hybrid orbitals either form sigma (σ) bonds directed toward other atoms of the molecule or contain lone pairs of electrons. We can determine the type of hybridization around a central atom from the geometry of the regions of electron density about it. Two such regions imply sp hybridization; three, sp2 hybridization; four, sp3 hybridization; five, sp3d hybridization; and six, sp3d2 hybridization. Pi (π) bonds are formed from unhybridized atomic orbitals (p or d orbitals).
Chapter 8.3: Multiple Bonds
Multiple bonds consist of a σ bond located along the axis between two atoms and one or two π bonds. The σ bonds are usually formed by the overlap of hybridized atomic orbitals, while the π bonds are formed by the side-by-side overlap of unhybridized orbitals. Resonance occurs when there are multiple unhybridized orbitals with the appropriate alignment to overlap, so the placement of π bonds can vary.
Chapter 8.4: Molecular Orbital Theory
Molecular orbital (MO) theory describes the behavior of electrons in a molecule in terms of combinations of the atomic wave functions. The resulting molecular orbitals may extend over all the atoms in the molecule. Bonding molecular orbitals are formed by in-phase combinations of atomic wave functions, and electrons in these orbitals stabilize a molecule. Antibonding molecular orbitals result from out-of-phase combinations of atomic wave functions and electrons in these orbitals make a molecule less stable. Molecular orbitals located along an internuclear axis are called σ MOs. They can be formed from s orbitals or from p orbitals oriented in an end-to-end fashion. Molecular orbitals formed from p orbitals oriented in a side-by-side fashion have electron density on opposite sides of the internuclear axis and are called π orbitals.
We can describe the electronic structure of diatomic molecules by applying molecular orbital theory to the valence electrons of the atoms. Electrons fill molecular orbitals following the same rules that apply to filling atomic orbitals; Hund’s rule and the Aufbau principle tell us that lower-energy orbitals will fill first, electrons will spread out before they pair up, and each orbital can hold a maximum of two electrons with opposite spins. Materials with unpaired electrons are paramagnetic and attracted to a magnetic field, while those with all-paired electrons are diamagnetic and repelled by a magnetic field. Correctly predicting the magnetic properties of molecules is in advantage of molecular orbital theory over Lewis structures and valence bond theory.
This content is available for free here
Chapter 9: Gases
Chapter 9.1: Gas Pressure
Gases exert pressure, which is force per unit area. The pressure of a gas may be expressed in the SI unit of pascal or kilopascal, as well as in many other units including torr, atmosphere, and bar. Atmospheric pressure is measured using a barometer; other gas pressures can be measured using one of several types of manometers.
Chapter 9.2: Relating Pressure, Volume, Amount, and Temperature: The Ideal Gas Law
The behavior of gases can be described by several laws based on experimental observations of their properties. The pressure of a given amount of gas is directly proportional to its absolute temperature, provided that the volume does not change (Amontons’s law). The volume of a given gas sample is directly proportional to its absolute temperature at constant pressure (Charles’s law). The volume of a given amount of gas is inversely proportional to its pressure when temperature is held constant (Boyle’s law). Under the same conditions of temperature and pressure, equal volumes of all gases contain the same number of molecules (Avogadro’s law).
The equations describing these laws are special cases of the ideal gas law, PV = nRT, where P is the pressure of the gas, V is its volume, n is the number of moles of the gas, T is its kelvin temperature, and R is the ideal (universal) gas constant.
Chapter 9.3: Stoichiometry of Gaseous Substances, Mixtures, and Reactions
The ideal gas law can be used to derive a number of convenient equations relating directly measured quantities to properties of interest for gaseous substances and mixtures. Appropriate rearrangement of the ideal gas equation may be made to permit the calculation of gas densities and molar masses. Dalton’s law of partial pressures may be used to relate measured gas pressures for gaseous mixtures to their compositions. Avogadro’s law may be used in stoichiometric computations for chemical reactions involving gaseous reactants or products.
Chapter 9.4: Effusion and Diffusion of Gases
Gaseous atoms and molecules move freely and randomly through space. Diffusion is the process whereby gaseous atoms and molecules are transferred from regions of relatively high concentration to regions of relatively low concentration. Effusion is a similar process in which gaseous species pass from a container to a vacuum through very small orifices. The rates of effusion of gases are inversely proportional to the square roots of their densities or to the square roots of their atoms/molecules’ masses (Graham’s law).
Chapter 9.5: The Kinetic-Molecular Theory
The kinetic molecular theory is a simple but very effective model that effectively explains ideal gas behavior. The theory assumes that gases consist of widely separated molecules of negligible volume that are in constant motion, colliding elastically with one another and the walls of their container with average velocities determined by their absolute temperatures. The individual molecules of a gas exhibit a range of velocities, the distribution of these velocities being dependent on the temperature of the gas and the mass of its molecules.
Chapter 9.6: Non-Ideal Gas Behavior
Gas molecules possess a finite volume and experience forces of attraction for one another. Consequently, gas behavior is not necessarily described well by the ideal gas law. Under conditions of low pressure and high temperature, these factors are negligible, the ideal gas equation is an accurate description of gas behavior, and the gas is said to exhibit ideal behavior. However, at lower temperatures and higher pressures, corrections for molecular volume and molecular attractions are required to account for finite molecular size and attractive forces. The van der Waals equation is a modified version of the ideal gas law that can be used to account for the non-ideal behavior of gases under these conditions.
This content is available for free here
Chapter 10: Liquids and Solids
Chapter 10.1: Intermolecular Forces
The physical properties of condensed matter (liquids and solids) can be explained in terms of the kinetic molecular theory. In a liquid, intermolecular attractive forces hold the molecules in contact, although they still have sufficient KE to move past each other.
Intermolecular attractive forces, collectively referred to as van der Waals forces, are responsible for the behavior of liquids and solids and are electrostatic in nature. Dipole-dipole attractions result from the electrostatic attraction of the partial negative end of one dipolar molecule for the partial positive end of another. The temporary dipole that results from the motion of the electrons in an atom can induce a dipole in an adjacent atom and give rise to the London dispersion force. London forces increase with increasing molecular size. Hydrogen bonds are a special type of dipole-dipole attraction that results when hydrogen is bonded to one of the three most electronegative elements: F, O, or N.
Chapter 10.2: Properties of Liquids
The intermolecular forces between molecules in the liquid state vary depending upon their chemical identities and result in corresponding variations in various physical properties. Cohesive forces between like molecules are responsible for a liquid’s viscosity (resistance to flow) and surface tension (elasticity of a liquid surface). Adhesive forces between the molecules of a liquid and different molecules composing a surface in contact with the liquid are responsible for phenomena such as surface wetting and capillary rise.
Chapter 10.3: Phase Transitions
Phase transitions are processes that convert matter from one physical state into another. There are six phase transitions between the three phases of matter. Melting, vaporization, and sublimation are all endothermic processes, requiring an input of heat to overcome intermolecular attractions. The reciprocal transitions of freezing, condensation, and deposition are all exothermic processes, involving heat as intermolecular attractive forces are established or strengthened. The temperatures at which phase transitions occur are determined by the relative strengths of intermolecular attractions and are, therefore, dependent on the chemical identity of the substance.
Chapter 10.4: Phase Diagrams
The temperature and pressure conditions at which a substance exists in solid, liquid, and gaseous states are summarized in a phase diagram for that substance. Phase diagrams are combined plots of three pressure-temperature equilibrium curves: solid-liquid, liquid-gas, and solid-gas. These curves represent the relationships between phase-transition temperatures and pressures. The point of intersection of all three curves represents the substance’s triple point—the temperature and pressure at which all three phases are in equilibrium. At pressures below the triple point, a substance cannot exist in the liquid state, regardless of its temperature. The terminus of the liquid-gas curve represents the substance’s critical point, the pressure and temperature above which a liquid phase cannot exist.
Chapter 10.5: The Solid State of Matter
Some substances form crystalline solids consisting of particles in a very organized structure; others form amorphous (noncrystalline) solids with an internal structure that is not ordered. The main types of crystalline solids are ionic solids, metallic solids, covalent network solids, and molecular solids. The properties of the different kinds of crystalline solids are due to the types of particles of which they consist, the arrangements of the particles, and the strengths of the attractions between them. Because their particles experience identical attractions, crystalline solids have distinct melting temperatures; the particles in amorphous solids experience a range of interactions, so they soften gradually and melt over a range of temperatures. Some crystalline solids have defects in the definite repeating pattern of their particles. These defects (which include vacancies, atoms or ions not in the regular positions, and impurities) change physical properties such as electrical conductivity, which is exploited in the silicon crystals used to manufacture computer chips.
Chapter 10.6: Lattice Structures in Crystalline Solids
The structures of crystalline metals and simple ionic compounds can be described in terms of packing of spheres. Metal atoms can pack in hexagonal closest-packed structures, cubic closest-packed structures, body-centered structures, and simple cubic structures. The anions in simple ionic structures commonly adopt one of these structures, and the cations occupy the spaces remaining between the anions. Small cations usually occupy tetrahedral holes in a closest-packed array of anions. Larger cations usually occupy octahedral holes. Still larger cations can occupy cubic holes in a simple cubic array of anions. The structure of a solid can be described by indicating the size and shape of a unit cell and the contents of the cell. The type of structure and dimensions of the unit cell can be determined by X-ray diffraction measurements.
This content is available for free here
Chapter 11: Solutions and Colloids
Chapter 11.1: The Dissolution Process
A solution forms when two or more substances combine physically to yield a mixture that is homogeneous at the molecular level. The solvent is the most concentrated component and determines the physical state of the solution. The solutes are the other components typically present at concentrations less than that of the solvent. Solutions may form endothermically or exothermically, depending upon the relative magnitudes of solute and solvent intermolecular attractive forces. Ideal solutions form with no appreciable change in energy.
Chapter 11.2: Electrolytes
Substances that dissolve in water to yield ions are called electrolytes. Electrolytes may be covalent compounds that chemically react with water to produce ions (for example, acids and bases), or they may be ionic compounds that dissociate to yield their constituent cations and anions, when dissolved. Dissolution of an ionic compound is facilitated by ion-dipole attractions between the ions of the compound and the polar water molecules. Soluble ionic substances and strong acids ionize completely and are strong electrolytes, while weak acids and bases ionize to only a small extent and are weak electrolytes. Nonelectrolytes are substances that do not produce ions when dissolved in water.
Chapter 11.3: Solubility
The extent to which one substance will dissolve in another is determined by several factors, including the types and relative strengths of intermolecular attractive forces that may exist between the substances’ atoms, ions, or molecules. This tendency to dissolve is quantified as substance’s solubility, its maximum concentration in a solution at equilibrium under specified conditions. A saturated solution contains solute at a concentration equal to its solubility. A supersaturated solution is one in which a solute’s concentration exceeds its solubility—a nonequilibrium (unstable) condition that will result in solute precipitation when the solution is appropriately perturbed. Miscible liquids are soluble in all proportions, and immiscible liquids exhibit very low mutual solubility. Solubilities for gaseous solutes decrease with increasing temperature, while those for most, but not all, solid solutes increase with temperature. The concentration of a gaseous solute in a solution is proportional to the partial pressure of the gas to which the solution is exposed, a relation known as Henry’s law.
Chapter 11.4: Colligative Properties
Properties of a solution that depend only on the concentration of solute particles are called colligative properties. They include changes in the vapor pressure, boiling point, and freezing point of the solvent in the solution. The magnitudes of these properties depend only on the total concentration of solute particles in solution, not on the type of particles. The total concentration of solute particles in a solution also determines its osmotic pressure. This is the pressure that must be applied to the solution to prevent diffusion of molecules of pure solvent through a semipermeable membrane into the solution. Ionic compounds may not completely dissociate in solution due to activity effects, in which case observed colligative effects may be less than predicted.
Chapter 11.5: Colloids
Colloids are mixtures in which one or more substances are dispersed as relatively large solid particles or liquid droplets throughout a solid, liquid, or gaseous medium. The particles of a colloid remain dispersed and do not settle due to gravity, and they are often electrically charged. Colloids are widespread in nature and are involved in many technological applications.
This content is available for free here
Chapter 12: Kinetics
Chapter 12.1: Chemical Reaction Rates
The rate of a reaction can be expressed either in terms of the decrease in the amount of a reactant or the increase in the amount of a product per unit time. Relations between different rate expressions for a given reaction are derived directly from the stoichiometric coefficients of the equation representing the reaction.
Chapter 12.2: Factors Affecting Reaction Rates
The rate of a chemical reaction is affected by several parameters. Reactions involving two phases proceed more rapidly when there is greater surface area contact. If temperature or reactant concentration is increased, the rate of a given reaction generally increases as well. A catalyst can increase the rate of a reaction by providing an alternative pathway that causes the activation energy of the reaction to decrease.
Chapter 12.3: Rate Laws
Rate laws provide a mathematical description of how changes in the amount of a substance affect the rate of a chemical reaction. Rate laws are determined experimentally and cannot be predicted by reaction stoichiometry. The order of reaction describes how much a change in the amount of each substance affects the overall rate, and the overall order of a reaction is the sum of the orders for each substance present in the reaction. Reaction orders are typically first order, second order, or zero order, but fractional and even negative orders are possible.
Chapter 12.4: Integrated Rate Laws
Differential rate laws can be determined by the method of initial rates or other methods. We measure values for the initial rates of a reaction at different concentrations of the reactants. From these measurements, we determine the order of the reaction in each reactant. Integrated rate laws are determined by integration of the corresponding differential rate laws. Rate constants for those rate laws are determined from measurements of concentration at various times during a reaction.
The half-life of a reaction is the time required to decrease the amount of a given reactant by one-half. The half-life of a zero-order reaction decreases as the initial concentration of the reactant in the reaction decreases. The half-life of a first-order reaction is independent of concentration, and the half-life of a second-order reaction decreases as the concentration increases.
Chapter 12.5: Collision Theory
Chemical reactions require collisions between reactant species. These reactant collisions must be of proper orientation and sufficient energy in order to result in product formation. Collision theory provides a simple but effective explanation for the effect of many experimental parameters on reaction rates. The Arrhenius equation describes the relation between a reaction’s rate constant and its activation energy, temperature, and dependence on collision orientation.
Chapter 12.6: Reaction Mechanisms
The sequence of individual steps, or elementary reactions, by which reactants are converted into products during the course of a reaction is called the reaction mechanism. The overall rate of a reaction is determined by the rate of the slowest step, called the rate-determining step. Unimolecular elementary reactions have first-order rate laws, while bimolecular elementary reactions have second-order rate laws. By comparing the rate laws derived from a reaction mechanism to that determined experimentally, the mechanism may be deemed either incorrect or plausible.
Chapter 12.7: Catalysis
Catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. Catalysts can be homogenous (in the same phase as the reactants) or heterogeneous (a different phase than the reactants).
This content is available for free here
Chapter 13: Fundamental Equilibrium Concepts
Chapter 13.1: Chemical Equilibria
A reaction is at equilibrium when the amounts of reactants or products no longer change. Chemical equilibrium is a dynamic process, meaning the rate of formation of products by the forward reaction is equal to the rate at which the products re-form reactants by the reverse reaction.
Chapter 13.2: Equilibrium Constants
For any reaction that is at equilibrium, the reaction quotient Q is equal to the equilibrium constant K for the reaction. If a reactant or product is a pure solid, a pure liquid, or the solvent in a dilute solution, the concentration of this component does not appear in the expression for the equilibrium constant. At equilibrium, the values of the concentrations of the reactants and products are constant. Their particular values may vary depending on conditions, but the value of the reaction quotient will always equal K (Kc when using concentrations or KP when using partial pressures).
A homogeneous equilibrium is an equilibrium in which all components are in the same phase. A heterogeneous equilibrium is an equilibrium in which components are in two or more phases. We can decide whether a reaction is at equilibrium by comparing the reaction quotient with the equilibrium constant for the reaction.
Chapter 13.3: Shifting Equilibria: Le Châtelier’s Principle
Systems at equilibrium can be disturbed by changes to temperature, concentration, and, in some cases, volume and pressure; volume and pressure changes will disturb equilibrium if the number of moles of gas is different on the reactant and product sides of the reaction. The system's response to these disturbances is described by Le Châtelier's principle: The system will respond in a way that counteracts the disturbance. Not all changes to the system result in a disturbance of the equilibrium. Adding a catalyst affects the rates of the reactions but does not alter the equilibrium, and changing pressure or volume will not significantly disturb systems with no gases or with equal numbers of moles of gas on the reactant and product side.
Effects of Disturbances of Equilibrium and K
Disturbance
Observed Change as Equilibrium is Restored
Direction of Shift
Effect on K
reactant added
added reactant is partially consumed
toward products
none
product added
added product is partially consumed
toward reactants
none
decrease in volume/increase in gas pressure
pressure decreases
toward side with fewer moles of gas
none
increase in volume/decrease in gas pressure
pressure increases
toward side with more moles of gas
none
temperature increase
heat is absorbed
toward products for endothermic, toward reactants for exothermic
changes
temperature decrease
heat is given off
toward reactants for endothermic, toward products for exothermic
changes
Chapter 13.4: Equilibrium Calculations
The ratios of the rate of change in concentrations of a reaction are equal to the ratios of the coefficients in the balanced chemical equation. The sign of the coefficient of X is positive when the concentration increases and negative when it decreases. We learned to approach three basic types of equilibrium problems. When given the concentrations of the reactants and products at equilibrium, we can solve for the equilibrium constant; when given the equilibrium constant and some of the concentrations involved, we can solve for the missing concentrations; and when given the equilibrium constant and the initial concentrations, we can solve for the concentrations at equilibrium.
This content is available for free here
Chapter 14: Acid-Base Equilibria
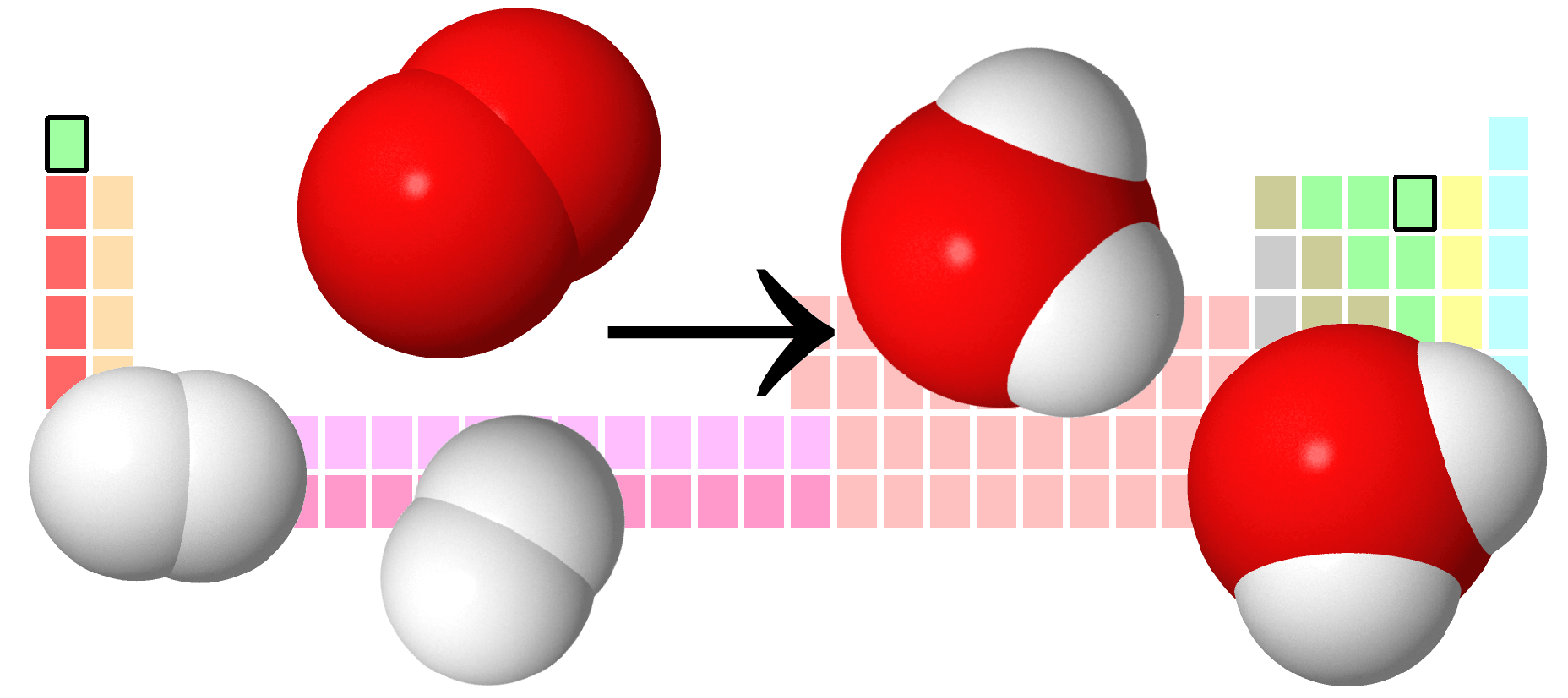
Chapter 14.1: Brønsted-Lowry Acids and Bases
A compound that can donate a proton (a hydrogen ion) to another compound is called a Brønsted-Lowry acid. The compound that accepts the proton is called a Brønsted-Lowry base. The species remaining after a Brønsted-Lowry acid has lost a proton is the conjugate base of the acid. The species formed when a Brønsted-Lowry base gains a proton is the conjugate acid of the base. Thus, an acid-base reaction occurs when a proton is transferred from an acid to a base, with formation of the conjugate base of the reactant acid and formation of the conjugate acid of the reactant base. Amphiprotic species can act as both proton donors and proton acceptors. Water is the most important amphiprotic species. It can form both the hydronium ion, H3O+, and the hydroxide ion, OH− when it undergoes autoionization:
The ion product of water, Kw is the equilibrium constant for the autoionization reaction:
Chapter 14.2: pH and pOH
The concentration of hydronium ion in a solution of an acid in water is greater than 1.0 10−7 M at 25 °C. The concentration of hydroxide ion in a solution of a base in water is greater than 1.0 10−7 M at 25 °C. The concentration of in a solution can be expressed as the pH of the solution; pH = −log The concentration of OH− can be expressed as the pOH of the solution: pOH = −log[OH−]. In pure water, pH = 7.00 and pOH = 7.00
Chapter 14.3: Relative Strengths of Acids and Bases
The strengths of Brønsted-Lowry acids and bases in aqueous solutions can be determined by their acid or base ionization constants. Stronger acids form weaker conjugate bases, and weaker acids form stronger conjugate bases. Thus strong acids are completely ionized in aqueous solution because their conjugate bases are weaker bases than water. Weak acids are only partially ionized because their conjugate bases are strong enough to compete successfully with water for possession of protons. Strong bases react with water to quantitatively form hydroxide ions. Weak bases give only small amounts of hydroxide ion. The strengths of the binary acids increase from left to right across a period of the periodic table (CH4 < NH3 < H2O < HF), and they increase down a group (HF < HCl < HBr < HI). The strengths of oxyacids that contain the same central element increase as the oxidation number of the element increases (H2SO3 < H2SO4). The strengths of oxyacids also increase as the electronegativity of the central element increases [H2SeO4 < H2SO4].
Chapter 14.4: Hydrolysis of Salt Solutions
The characteristic properties of aqueous solutions of Brønsted-Lowry acids are due to the presence of hydronium ions; those of aqueous solutions of Brønsted-Lowry bases are due to the presence of hydroxide ions. The neutralization that occurs when aqueous solutions of acids and bases are combined results from the reaction of the hydronium and hydroxide ions to form water. Some salts formed in neutralization reactions may make the product solutions slightly acidic or slightly basic.
Solutions that contain salts or hydrated metal ions have a pH that is determined by the extent of the hydrolysis of the ions in the solution. The pH of the solutions may be calculated using familiar equilibrium techniques, or it may be qualitatively determined to be acidic, basic, or neutral depending on the relative Ka and Kb of the ions involved.
Chapter 14.5: Polyprotic Acids
An acid that contains more than one ionizable proton is a polyprotic acid. The protons of these acids ionize in steps. The differences in the acid ionization constants for the successive ionizations of the protons in a polyprotic acid usually vary by roughly five orders of magnitude. As long as the difference between the successive values of Ka of the acid is greater than about a factor of 20, it is appropriate to break down the calculations of the concentrations of the ions in solution into a series of steps.
Chapter 14.6: Buffers
A solution containing a mixture of an acid and its conjugate base, or of a base and its conjugate acid, is called a buffer solution. Unlike in the case of an acid, base, or salt solution, the hydronium ion concentration of a buffer solution does not change greatly when a small amount of acid or base is added to the buffer solution. The base (or acid) in the buffer reacts with the added acid (or base).
Chapter 14.7: Acid-Base Titrations
A titration curve is a graph that relates the change in pH of an acidic or basic solution to the volume of added titrant. The characteristics of the titration curve are dependent on the specific solutions being titrated. The pH of the solution at the equivalence point may be greater than, equal to, or less than 7.00. The choice of an indicator for a given titration depends on the expected pH at the equivalence point of the titration, and the range of the color change of the indicator.
This content is available for free here
Chapter 15: Equilibria of Other Reaction Classes
Chapter 15.1: Precipitation and Dissolution
The equilibrium constant for an equilibrium involving the precipitation or dissolution of a slightly soluble ionic solid is called the solubility product, Ksp, of the solid. When we have a heterogeneous equilibrium involving the slightly soluble solid MpXq and its ions Mm+ and Xn–:
We write the solubility product expression as:
The solubility product of a slightly soluble electrolyte can be calculated from its solubility; conversely, its solubility can be calculated from its Ksp, provided the only significant reaction that occurs when the solid dissolves is the formation of its ions.
A slightly soluble electrolyte begins to precipitate when the magnitude of the reaction quotient for the dissolution reaction exceeds the magnitude of the solubility product. Precipitation continues until the reaction quotient equals the solubility product.
A reagent can be added to a solution of ions to allow one ion to selectively precipitate out of solution. The common ion effect can also play a role in precipitation reactions. In the presence of an ion in common with one of the ions in the solution, Le Châtelier’s principle applies and more precipitate comes out of solution so that the molar solubility is reduced.
Chapter 15.2: Lewis Acids and Bases
G.N. Lewis proposed a definition for acids and bases that relies on an atom’s or molecule’s ability to accept or donate electron pairs. A Lewis acid is a species that can accept an electron pair, whereas a Lewis base has an electron pair available for donation to a Lewis acid. Complex ions are examples of Lewis acid-base adducts. In a complex ion, we have a central atom, often consisting of a transition metal cation, which acts as a Lewis acid, and several neutral molecules or ions surrounding them called ligands that act as Lewis bases. Complex ions form by sharing electron pairs to form coordinate covalent bonds. The equilibrium reaction that occurs when forming a complex ion has an equilibrium constant associated with it called a formation constant, Kf. This is often referred to as a stability constant, as it represents the stability of the complex ion. Formation of complex ions in solution can have a profound effect on the solubility of a transition metal compound.
Chapter 15.3: Multiple Equilibria
Several systems we encounter consist of multiple equilibria, systems where two or more equilibria processes are occurring simultaneously. Some common examples include acid rain, fluoridation, and dissolution of carbon dioxide in sea water. When looking at these systems, we need to consider each equilibrium separately and then combine the individual equilibrium constants into one solubility product or reaction quotient expression using the tools from the first equilibrium chapter. Le Châtelier’s principle also must be considered, as each reaction in a multiple equilibria system will shift toward reactants or products based on what is added to the initial reaction and how it affects each subsequent equilibrium reaction.
This content is available for free here
Chapter 16: Thermodynamics
Chapter 16.1: Spontaneity
Chemical and physical processes have a natural tendency to occur in one direction under certain conditions. A spontaneous process occurs without the need for a continual input of energy from some external source, while a nonspontaneous process requires such. Systems undergoing a spontaneous process may or may not experience a gain or loss of energy, but they will experience a change in the way matter and/or energy is distributed within the system.
Chapter 16.2: Entropy
Entropy (S) is a state function that can be related to the number of microstates for a system (the number of ways the system can be arranged) and to the ratio of reversible heat to kelvin temperature. It may be interpreted as a measure of the dispersal or distribution of matter and/or energy in a system, and it is often described as representing the “disorder” of the system.
For a given substance, Ssolid < Sliquid < Sgas in a given physical state at a given temperature, entropy is typically greater for heavier atoms or more complex molecules. Entropy increases when a system is heated and when solutions form. Using these guidelines, the sign of entropy changes for some chemical reactions may be reliably predicted.
Chapter 16.3: The Second and Third Laws of Thermodynamics
The second law of thermodynamics states that a spontaneous process increases the entropy of the universe, Suniv > 0. If ΔSuniv < 0, the process is nonspontaneous, and if ΔSuniv = 0, the system is at equilibrium. The third law of thermodynamics establishes the zero for entropy as that of a perfect, pure crystalline solid at 0 K. With only one possible microstate, the entropy is zero. We may compute the standard entropy change for a process by using standard entropy values for the reactants and products involved in the process.
Chapter 16.4: Free Energy
Gibbs free energy (G) is a state function defined with regard to system quantities only and may be used to predict the spontaneity of a process. A negative value for ΔG indicates a spontaneous process; a positive ΔG indicates a nonspontaneous process; and a ΔG of zero indicates that the system is at equilibrium. A number of approaches to the computation of free energy changes are possible.
This content is available for free here
Chapter 17: Electrochemistry
Chapter 17.1: Balancing Oxidation-Reduction Reactions
An electric current consists of moving charge. The charge may be in the form of electrons or ions. Current flows through an unbroken or closed circular path called a circuit. The current flows through a conducting medium as a result of a difference in electrical potential between two points in a circuit. Electrical potential has the units of energy per charge. In SI units, charge is measured in coulombs (C), current in amperes and electrical potential in volts
Oxidation is the loss of electrons, and the species that is oxidized is also called the reducing agent. Reduction is the gain of electrons, and the species that is reduced is also called the oxidizing agent. Oxidation-reduction reactions can be balanced using the half-reaction method. In this method, the oxidation-reduction reaction is split into an oxidation half-reaction and a reduction half-reaction. The oxidation half-reaction and reduction half-reaction are then balanced separately. Each of the half-reactions must have the same number of each type of atom on both sides of the equation and show the same total charge on each side of the equation. Charge is balanced in oxidation half-reactions by adding electrons as products; in reduction half-reactions, charge is balanced by adding electrons as reactants. The total number of electrons gained by reduction must exactly equal the number of electrons lost by oxidation when combining the two half-reactions to give the overall balanced equation. Balancing oxidation-reduction reaction equations in aqueous solutions frequently requires that oxygen or hydrogen be added or removed from a reactant. In acidic solution, hydrogen is added by adding hydrogen ion (H+) and removed by producing hydrogen ion; oxygen is removed by adding hydrogen ion and producing water, and added by adding water and producing hydrogen ion. A balanced equation in basic solution can be obtained by first balancing the equation in acidic solution, and then adding hydroxide ion to each side of the balanced equation in such numbers that all the hydrogen ions are converted to water.
Chapter 17.2: Galvanic Cells
Electrochemical cells typically consist of two half-cells. The half-cells separate the oxidation half-reaction from the reduction half-reaction and make it possible for current to flow through an external wire. One half-cell, normally depicted on the left side in a figure, contains the anode. Oxidation occurs at the anode. The anode is connected to the cathode in the other half-cell, often shown on the right side in a figure. Reduction occurs at the cathode. Adding a salt bridge completes the circuit allowing current to flow. Anions in the salt bridge flow toward the anode and cations in the salt bridge flow toward the cathode. The movement of these ions completes the circuit and keeps each half-cell electrically neutral. Electrochemical cells can be described using cell notation. In this notation, information about the reaction at the anode appears on the left and information about the reaction at the cathode on the right. The salt bridge is represented by a double line, ‖. The solid, liquid, or aqueous phases within a half-cell are separated by a single line, │. The phase and concentration of the various species is included after the species name. Electrodes that participate in the oxidation-reduction reaction are called active electrodes. Electrodes that do not participate in the oxidation-reduction reaction but are there to allow current to flow are inert electrodes. Inert electrodes are often made from platinum or gold, which are unchanged by many chemical reactions.
Chapter 17.3: Standard Reduction Potentials
Assigning the potential of the standard hydrogen electrode (SHE) as zero volts allows the determination of standard reduction potentials, E°, for half-reactions in electrochemical cells. As the name implies, standard reduction potentials use standard states (1 bar or 1 atm for gases; 1 M for solutes, often at 298.15 K) and are written as reductions (where electrons appear on the left side of the equation). The reduction reactions are reversible, so standard cell potentials can be calculated by subtracting the standard reduction potential for the reaction at the anode from the standard reduction for the reaction at the cathode. When calculating the standard cell potential, the standard reduction potentials are not scaled by the stoichiometric coefficients in the balanced overall equation.
Chapter 17.4: The Nernst Equation
Electrical work (wele) is the negative of the product of the total charge (Q) and the cell potential (Ecell). The total charge can be calculated as the number of moles of electrons (n) times the Faraday constant (F = 96,485 C/mol e−). Electrical work is the maximum work that the system can produce and so is equal to the change in free energy. Thus, anything that can be done with or to a free energy change can also be done to or with a cell potential. The Nernst equation relates the cell potential at nonstandard conditions to the logarithm of the reaction quotient. Concentration cells exploit this relationship and produce a positive cell potential using half-cells that differ only in the concentration of their solutes.
Chapter 17.5: Batteries and Fuel Cells
Batteries are galvanic cells, or a series of cells, that produce an electric current. When cells are combined into batteries, the potential of the battery is an integer multiple of the potential of a single cell. There are two basic types of batteries: primary and secondary. Primary batteries are “single use” and cannot be recharged. Dry cells and (most) alkaline batteries are examples of primary batteries. The second type is rechargeable and is called a secondary battery. Examples of secondary batteries include nickel-cadmium (NiCd), lead acid, and lithium ion batteries. Fuel cells are similar to batteries in that they generate an electrical current, but require continuous addition of fuel and oxidizer. The hydrogen fuel cell uses hydrogen and oxygen from the air to produce water, and is generally more efficient than internal combustion engines.
Chapter 17.6: Corrosion
Corrosion is the degradation of a metal caused by an electrochemical process. Large sums of money are spent each year repairing the effects of, or preventing, corrosion. Some metals, such as aluminum and copper, produce a protective layer when they corrode in air. The thin layer that forms on the surface of the metal prevents oxygen from coming into contact with more of the metal atoms and thus “protects” the remaining metal from further corrosion. Iron corrodes (forms rust) when exposed to water and oxygen. The rust that forms on iron metal flakes off, exposing fresh metal, which also corrodes. One way to prevent, or slow, corrosion is by coating the metal. Coating prevents water and oxygen from contacting the metal. Paint or other coatings will slow corrosion, but they are not effective once scratched. Zinc-plated or galvanized iron exploits the fact that zinc is more likely to oxidize than iron. As long as the coating remains, even if scratched, the zinc will oxidize before the iron. Another method for protecting metals is cathodic protection. In this method, an easily oxidized and inexpensive metal, often zinc or magnesium (the sacrificial anode), is electrically connected to the metal that must be protected. The more active metal is the sacrificial anode, and is the anode in a galvanic cell. The “protected” metal is the cathode, and remains unoxidized. One advantage of cathodic protection is that the sacrificial anode can be monitored and replaced if needed.
Chapter 17.7: Electrolysis
Using electricity to force a nonspontaneous process to occur is electrolysis. Electrolytic cells are electrochemical cells with negative cell potentials (meaning a positive Gibbs free energy), and so are nonspontaneous. Electrolysis can occur in electrolytic cells by introducing a power supply, which supplies the energy to force the electrons to flow in the nonspontaneous direction. Electrolysis is done in solutions, which contain enough ions so current can flow. If the solution contains only one material, like the electrolysis of molten sodium chloride, it is a simple matter to determine what is oxidized and what is reduced. In more complicated systems, like the electrolysis of aqueous sodium chloride, more than one species can be oxidized or reduced and the standard reduction potentials are used to determine the most likely oxidation (the half-reaction with the largest [most positive] standard reduction potential) and reduction (the half-reaction with the smallest [least positive] standard reduction potential). Sometimes unexpected half-reactions occur because of overpotential. Overpotential is the difference between the theoretical half-reaction reduction potential and the actual voltage required. When present, the applied potential must be increased, making it possible for a different reaction to occur in the electrolytic cell. The total charge, Q, that passes through an electrolytic cell can be expressed as the current (I) multiplied by time (Q = It) or as the moles of electrons (n) multiplied by Faraday’s constant (Q = nF). These relationships can be used to determine things like the amount of material used or generated during electrolysis, how long the reaction must proceed, or what value of the current is required.
This content is available for free here
Chapter 18: Representative Metals, Metalloids, and Nonmetals
Chapter 18.1: Periodicity
This section focuses on the periodicity of the representative elements. These are the elements where the electrons are entering the s and p orbitals. The representative elements occur in groups 1, 2, and 12–18. These elements are representative metals, metalloids, and nonmetals. The alkali metals (group 1) are very reactive, readily form ions with a charge of 1+ to form ionic compounds that are usually soluble in water, and react vigorously with water to form hydrogen gas and a basic solution of the metal hydroxide. The outermost electrons of the alkaline earth metals (group 2) are more difficult to remove than the outer electron of the alkali metals, leading to the group 2 metals being less reactive than those in group 1. These elements easily form compounds in which the metals exhibit an oxidation state of 2+. Zinc, cadmium, and mercury (group 12) commonly exhibit the group oxidation state of 2+ (although mercury also exhibits an oxidation state of 1+ in compounds that contain Aluminum, gallium, indium, and thallium (group 13) are easier to oxidize than is hydrogen. Aluminum, gallium, and indium occur with an oxidation state 3+ (however, thallium also commonly occurs as the Tl+ ion). Tin and lead form stable divalent cations and covalent compounds in which the metals exhibit the 4+-oxidation state.
Chapter 18.2: Occurrence and Preparation of the Representative Metals
Because of their chemical reactivity, it is necessary to produce the representative metals in their pure forms by reduction from naturally occurring compounds. Electrolysis is important in the production of sodium, potassium, and aluminum. Chemical reduction is the primary method for the isolation of magnesium, zinc, and tin. Similar procedures are important for the other representative metals.
Chapter 18.3: Structure and General Properties of the Metalloids
The elements boron, silicon, germanium, arsenic, antimony, and tellurium separate the metals from the nonmetals in the periodic table. These elements, called metalloids or sometimes semimetals, exhibit properties characteristic of both metals and nonmetals. The structures of these elements are similar in many ways to those of nonmetals, but the elements are electrical semiconductors.
Chapter 18.4: Structure and General Properties of the Nonmetals
Nonmetals have structures that are very different from those of the metals, primarily because they have greater electronegativity and electrons that are more tightly bound to individual atoms. Most nonmetal oxides are acid anhydrides, meaning that they react with water to form acidic solutions. Molecular structures are common for most of the nonmetals, and several have multiple allotropes with varying physical properties.
Chapter 18.5: Occurrence, Preparation, and Compounds of Hydrogen
Hydrogen is the most abundant element in the universe and its chemistry is truly unique. Although it has some chemical reactivity that is similar to that of the alkali metals, hydrogen has many of the same chemical properties of a nonmetal with a relatively low electronegativity. It forms ionic hydrides with active metals, covalent compounds in which it has an oxidation state of 1− with less electronegative elements, and covalent compounds in which it has an oxidation state of 1+ with more electronegative nonmetals. It reacts explosively with oxygen, fluorine, and chlorine, less readily with bromine, and much less readily with iodine, sulfur, and nitrogen. Hydrogen reduces the oxides of metals with lower reduction potentials than chromium to form the metal and water. The hydrogen halides are all acidic when dissolved in water.
Chapter 18.6: Occurrence, Preparation, and Properties of Carbonates
The usual method for the preparation of the carbonates of the alkali and alkaline earth metals is by reaction of an oxide or hydroxide with carbon dioxide. Other carbonates form by precipitation. Metal carbonates or hydrogen carbonates such as limestone (CaCO3), the antacid Tums (CaCO3), and baking soda (NaHCO3) are common examples. Carbonates and hydrogen carbonates decompose in the presence of acids and most decompose on heating.
Chapter 18.7: Occurrence, Preparation, and Properties of Nitrogen
Nitrogen exhibits oxidation states ranging from 3− to 5+. Because of the stability of the N≡N triple bond, it requires a great deal of energy to make compounds from molecular nitrogen. Active metals such as the alkali metals and alkaline earth metals can reduce nitrogen to form metal nitrides. Nitrogen oxides and nitrogen hydrides are also important substances.
Chapter 18.8: Occurrence, Preparation, and Properties of Phosphorus
Phosphorus (group 15) commonly exhibits oxidation states of 3− with active metals and of 3+ and 5+ with more electronegative nonmetals. The halogens and oxygen will oxidize phosphorus. The oxides are phosphorus(V) oxide, P4O10, and phosphorus(III) oxide, P4O6. The two common methods for preparing orthophosphoric acid, H3PO4, are either the reaction of a phosphate with sulfuric acid or the reaction of water with phosphorus(V) oxide. Orthophosphoric acid is a triprotic acid that forms three types of salts.
Chapter 18.9: Occurrence, Preparation, and Compounds of Oxygen
Oxygen is one of the most reactive elements. This reactivity, coupled with its abundance, makes the chemistry of oxygen very rich and well understood.
Compounds of the representative metals with oxygen exist in three categories (1) oxides, (2) peroxides and superoxides, and (3) hydroxides. Heating the corresponding hydroxides, nitrates, or carbonates is the most common method for producing oxides. Heating the metal or metal oxide in oxygen may lead to the formation of peroxides and superoxides. The soluble oxides dissolve in water to form solutions of hydroxides. Most metals oxides are base anhydrides and react with acids. The hydroxides of the representative metals react with acids in acid-base reactions to form salts and water. The hydroxides have many commercial uses.
All nonmetals except fluorine form multiple oxides. Nearly all of the nonmetal oxides are acid anhydrides. The acidity of oxyacids requires that the hydrogen atoms bond to the oxygen atoms in the molecule rather than to the other nonmetal atom. Generally, the strength of the oxyacid increases with the number of oxygen atoms bonded to the nonmetal atom and not to a hydrogen.
Chapter 18.10: Occurrence, Preparation, and Properties of Sulfur
Sulfur (group 16) reacts with almost all metals and readily forms the sulfide ion, S2−, in which it has as oxidation state of 2−. Sulfur reacts with most nonmetals.
Chapter 18.11: Occurrence, Preparation, and Properties of Halogens
The halogens form halides with less electronegative elements. Halides of the metals vary from ionic to covalent; halides of nonmetals are covalent. Interhalogens form by the combination of two or more different halogens.
All of the representative metals react directly with elemental halogens or with solutions of the hydrohalic acids (HF, HCl, HBr, and HI) to produce representative metal halides. Other laboratory preparations involve the addition of aqueous hydrohalic acids to compounds that contain such basic anions, such as hydroxides, oxides, or carbonates.
Chapter 18.12: Occurrence, Preparation, and Properties of the Noble Gases
The most significant property of the noble gases (group 18) is their inactivity. They occur in low concentrations in the atmosphere. They find uses as inert atmospheres, neon signs, and as coolants. The three heaviest noble gases react with fluorine to form fluorides. The xenon fluorides are the best characterized as the starting materials for a few other noble gas compounds.
This content is available for free here
Chapter 19: Transition Metals and Coordination Chemistry
Chapter 19.1: Occurrence, Preparation, and Properties of Transition Metals and Their Compounds
The transition metals are elements with partially filled d orbitals, located in the d-block of the periodic table. The reactivity of the transition elements varies widely from very active metals such as scandium and iron to almost inert elements, such as the platinum metals. The type of chemistry used in the isolation of the elements from their ores depends upon the concentration of the element in its ore and the difficulty of reducing ions of the elements to the metals. Metals that are more active are more difficult to reduce.
Transition metals exhibit chemical behavior typical of metals. For example, they oxidize in air upon heating and react with elemental halogens to form halides. Those elements that lie above hydrogen in the activity series react with acids, producing salts and hydrogen gas. Oxides, hydroxides, and carbonates of transition metal compounds in low oxidation states are basic. Halides and other salts are generally stable in water, although oxygen must be excluded in some cases. Most transition metals form a variety of stable oxidation states, allowing them to demonstrate a wide range of chemical reactivity.
Chapter 19.2: Coordination Chemistry of Transition Metals
The transition elements and main group elements can form coordination compounds, or complexes, in which a central metal atom or ion is bonded to one or more ligands by coordinate covalent bonds. Ligands with more than one donor atom are called polydentate ligands and form chelates. The common geometries found in complexes are tetrahedral and square planar (both with a coordination number of four) and octahedral (with a coordination number of six). Cis and trans configurations are possible in some octahedral and square planar complexes. In addition to these geometrical isomers, optical isomers (molecules or ions that are mirror images but not superimposable) are possible in certain octahedral complexes. Coordination complexes have a wide variety of uses including oxygen transport in blood, water purification, and pharmaceutical use.
Chapter 19.3: Spectroscopic and Magnetic Properties of Coordination Compounds
Crystal field theory treats interactions between the electrons on the metal and the ligands as a simple electrostatic effect. The presence of the ligands near the metal ion changes the energies of the metal d orbitals relative to their energies in the free ion. Both the color and the magnetic properties of a complex can be attributed to this crystal field splitting. The magnitude of the splitting (Δoct) depends on the nature of the ligands bonded to the metal. Strong-field ligands produce large splitting and favor low-spin complexes, in which the t2g orbitals are completely filled before any electrons occupy the eg orbitals. Weak-field ligands favor formation of high-spin complexes. The t2g and the eg orbitals are singly occupied before any are doubly occupied.
This content is available for free here
Chapter 20: Organic Chemistry
Chapter 20.1: Hydrocarbons
Strong, stable bonds between carbon atoms produce complex molecules containing chains, branches, and rings. The chemistry of these compounds is called organic chemistry. Hydrocarbons are organic compounds composed of only carbon and hydrogen. The alkanes are saturated hydrocarbons—that is, hydrocarbons that contain only single bonds. Alkenes contain one or more carbon-carbon double bonds. Alkynes contain one or more carbon-carbon triple bonds. Aromatic hydrocarbons contain ring structures with delocalized π electron systems.
Chapter 20.2: Alcohols and Ethers
Many organic compounds that are not hydrocarbons can be thought of as derivatives of hydrocarbons. A hydrocarbon derivative can be formed by replacing one or more hydrogen atoms of a hydrocarbon by a functional group, which contains at least one atom of an element other than carbon or hydrogen. The properties of hydrocarbon derivatives are determined largely by the functional group. The –OH group is the functional group of an alcohol. The –R–O–R– group is the functional group of an ether.
Chapter 20.3: Aldehydes, Ketones, Carboxylic Acids, and Esters
Functional groups related to the carbonyl group include the –CHO group of an aldehyde, the –CO– group of a ketone, the –CO2H group of a carboxylic acid, and the –CO2R group of an ester. The carbonyl group, a carbon-oxygen double bond, is the key structure in these classes of organic molecules: Aldehydes contain at least one hydrogen atom attached to the carbonyl carbon atom, ketones contain two carbon groups attached to the carbonyl carbon atom, carboxylic acids contain a hydroxyl group attached to the carbonyl carbon atom, and esters contain an oxygen atom attached to another carbon group connected to the carbonyl carbon atom. All of these compounds contain oxidized carbon atoms relative to the carbon atom of an alcohol group.
Chapter 20.4: Amines and Amides
The addition of nitrogen into an organic framework leads to two families of molecules. Compounds containing a nitrogen atom bonded in a hydrocarbon framework are classified as amines. Compounds that have a nitrogen atom bonded to one side of a carbonyl group are classified as amides. Amines are a basic functional group. Amines and carboxylic acids can combine in a condensation reaction to form amides.
This content is available for free here
Chapter 21: Nuclear Chemistry
Chapter 21.1: Nuclear Structure and Stability
An atomic nucleus consists of protons and neutrons, collectively called nucleons. Although protons repel each other, the nucleus is held tightly together by a short-range, but very strong, force called the strong nuclear force. A nucleus has less mass than the total mass of its constituent nucleons. This “missing” mass is the mass defect, which has been converted into the binding energy that holds the nucleus together according to Einstein’s mass-energy equivalence equation, E = mc2. Of the many nuclides that exist, only a small number are stable. Nuclides with even numbers of protons or neutrons, or those with magic numbers of nucleons, are especially likely to be stable. These stable nuclides occupy a narrow band of stability on a graph of number of protons versus number of neutrons. The binding energy per nucleon is largest for the elements with mass numbers near 56; these are the most stable nuclei.
Chapter 21.2: Nuclear Equations
Nuclei can undergo reactions that change their number of protons, number of neutrons, or energy state. Many different particles can be involved in nuclear reactions. The most common are protons, neutrons, positrons (which are positively charged electrons), alpha (α) particles (which are high-energy helium nuclei), beta (β) particles (which are high-energy electrons), and gamma (γ) rays (which compose high-energy electromagnetic radiation). As with chemical reactions, nuclear reactions are always balanced. When a nuclear reaction occurs, the total mass (number) and the total charge remain unchanged.
Chapter 21.3: Radioactive Decay
Nuclei that have unstable n:p ratios undergo spontaneous radioactive decay. The most common types of radioactivity are α decay, β decay, γ emission, positron emission, and electron capture. Nuclear reactions also often involve γ rays, and some nuclei decay by electron capture. Each of these modes of decay leads to the formation of a new nucleus with a more stable n:p ratio. Some substances undergo radioactive decay series, proceeding through multiple decays before ending in a stable isotope. All nuclear decay processes follow first-order kinetics, and each radioisotope has its own characteristic half-life, the time that is required for half of its atoms to decay. Because of the large differences in stability among nuclides, there is a very wide range of half-lives of radioactive substances. Many of these substances have found useful applications in medical diagnosis and treatment, determining the age of archaeological and geological objects, and more.
Chapter 21.4: Transmutation and Nuclear Energy
It is possible to produce new atoms by bombarding other atoms with nuclei or high-speed particles. The products of these transmutation reactions can be stable or radioactive. A number of artificial elements, including technetium, astatine, and the transuranium elements, have been produced in this way.
Nuclear power as well as nuclear weapon detonations can be generated through fission (reactions in which a heavy nucleus is split into two or more lighter nuclei and several neutrons). Because the neutrons may induce additional fission reactions when they combine with other heavy nuclei, a chain reaction can result. Useful power is obtained if the fission process is carried out in a nuclear reactor. The conversion of light nuclei into heavier nuclei (fusion) also produces energy. At present, this energy has not been contained adequately and is too expensive to be feasible for commercial energy production.
Chapter 21.5: Uses of Radioisotopes
Compounds known as radioactive tracers can be used to follow reactions, track the distribution of a substance, diagnose and treat medical conditions, and much more. Other radioactive substances are helpful for controlling pests, visualizing structures, providing fire warnings, and for many other applications. Hundreds of millions of nuclear medicine tests and procedures, using a wide variety of radioisotopes with relatively short half-lives, are performed every year in the US. Most of these radioisotopes have relatively short half-lives; some are short enough that the radioisotope must be made on-site at medical facilities. Radiation therapy uses high-energy radiation to kill cancer cells by damaging their DNA. The radiation used for this treatment may be delivered externally or internally.
Chapter 21.6: Biological Effects of Radiation
We are constantly exposed to radiation from a variety of naturally occurring and human-produced sources. This radiation can affect living organisms. Ionizing radiation is the most harmful because it can ionize molecules or break chemical bonds, which damages the molecule and causes malfunctions in cell processes. It can also create reactive hydroxyl radicals that damage biological molecules and disrupt physiological processes. Radiation can cause somatic or genetic damage, and is most harmful to rapidly reproducing cells. Types of radiation differ in their ability to penetrate material and damage tissue, with alpha particles the least penetrating but potentially most damaging and gamma rays the most penetrating.
Various devices, including Geiger counters, scintillators, and dosimeters, are used to detect and measure radiation, and monitor radiation exposure. We use several units to measure radiation: becquerels or curies for rates of radioactive decay; gray or rads for energy absorbed; and rems or sieverts for biological effects of radiation. Exposure to radiation can cause a wide range of health effects, from minor to severe, and including death. We can minimize the effects of radiation by shielding with dense materials such as lead, moving away from the source, and limiting time of exposure.


