Example 7.4: Writing Lewis Structures
NASA’s Cassini-Huygens mission detected a large cloud of toxic hydrogen cyanide \(\ce{(HCN)}\) on Titan, one of Saturn’s moons. Titan also contains ethane \(\ce{(H3CCH3)}\), acetylene \(\ce{(HCCH)}\), and ammonia \(\ce{(NH3)}\). What are the Lewis structures of these molecules?Solution
Calculate the number of valence electrons.\(\mathrm{VE}_{\mathrm{\ce{HCN}}}\) \(= 1 \cdot 1 + 1 \cdot 4 + 1 \cdot 5\)
\(\ \ \ =1 + 4 + 5\)
\(\ \ \ =5 + 5\)
\(\ \ \ =10\)
\(\mathrm{VE}_{\mathrm{\ce{H3CCH3}}}\) \(= 3 \cdot 1 + 2 \cdot 4 + 3 \cdot 1\)
\(\ \ \ =3 + 8 + 3\)
\(\ \ \ =11 + 3\)
\(\ \ \ =14\)
\(\mathrm{VE}_{\mathrm{\ce{HCCH}}}\) \(= 1 \cdot 1 + 2 \cdot 4 + 1 \cdot 1\)
\(\ \ \ =1 + 8 + 1\)
\(\ \ \ =9 + 1\)
\(\ \ \ =10\)
\(\mathrm{VE}_{\mathrm{\ce{NH3}}}\) \(= 1 \cdot 5 + 1 \cdot 3\)
\(\ \ \ =5 + 3\)
\(\ \ \ =8\)
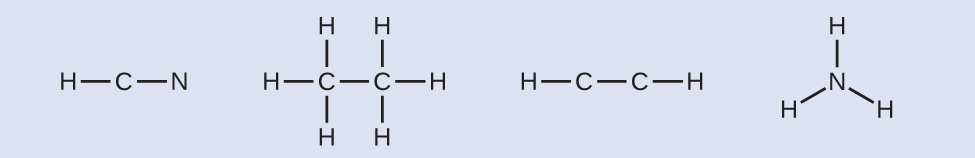
Draw a skeleton and connect the atoms with single bonds. Remember that H is never a central atom:
Where needed, distribute electrons to the terminal atoms (don't use more electrons than available, though):
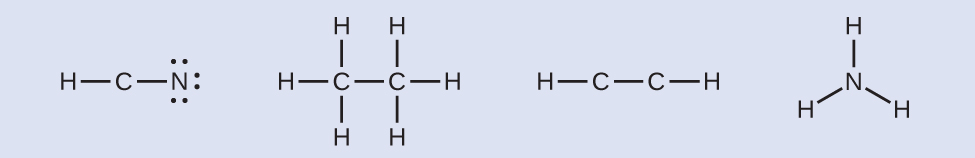
HCN: six electrons placed on N
\(\ce{H3CCH3:}\) no electrons remain and no terminal atoms capable of accepting electrons
HCCH: no terminal atoms capable of accepting electrons
\(\ce{NH3:}\) no terminal atoms capable of accepting electrons
Where needed, place remaining electrons on the central atom:
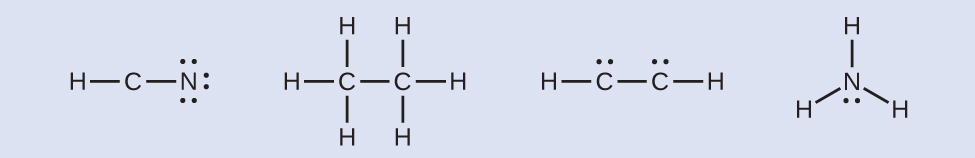
HCN: no electrons remain
\(\ce{H3CCH3:}\) no electrons remain
HCCH: four remaining electrons placed on carbon
\(\ce{NH3:}\) two remaining electrons placed on nitrogen
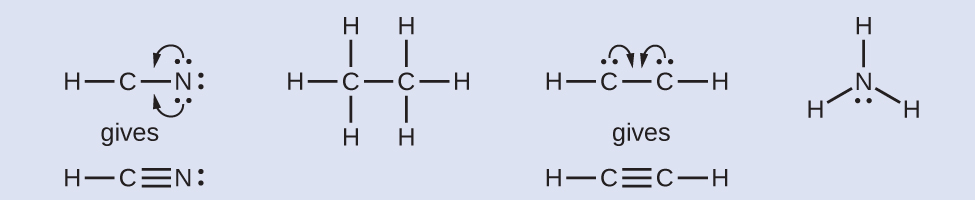
Where needed, rearrange electrons to form multiple bonds in order to obtain an octet on each atom:
HCN: form two more C–N bonds
\(\ce{H3CCH3:}\) all atoms have the correct number of electrons
HCCH: form a triple bond between the two carbon atoms
\(\ce{NH3:}\) all atoms have the correct number of electrons