Example 7.6: Calculating Formal Charge from Lewis Structures
Assign formal charges to each atom in the interhalogen ion \(\ce{ICl4-}\).Solution
We divide the bonding electron pairs equally for all I–Cl bonds: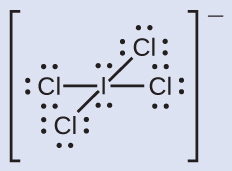
We assign lone pairs of electrons to their atoms. Each Cl atom now has seven electrons assigned to it, and the I atom has eight.
Subtract this number from the number of valence electrons for the neutral atom:
\(\mathrm{FC}_{\mathrm{\ce{I}}}\) \(= 7 - 8\)
\(\ \ \ =-1\)
\(\mathrm{FC}_{\mathrm{\ce{Cl}}}\) \(= 7 - 7\)
\(\ \ \ =0\)
The sum of the formal charges of all the atoms equals -1, which is identical to the charge of the ion (-1).