Example 9.4: Calculation of Pressure Using an Open-End Manometer
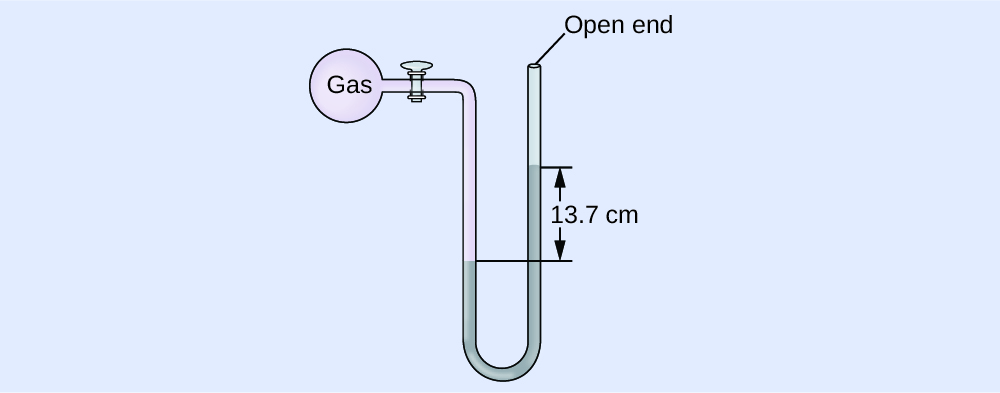
The pressure of a sample of gas is measured at sea level with an open-end Hg (mercury) manometer, as shown below. Determine the pressure of the gas in:(a) mm Hg
(b) atm
(c) kPa
Solution
\(ΔP\) \(= 137.\ \mathrm{mmHg}\)The pressure of the gas equals the hydrostatic pressure due to a column of mercury of height 13.7 cm plus the pressure of the atmosphere at sea level. (The pressure at the bottom horizontal line is equal on both sides of the tube. The pressure on the left is due to the gas and the pressure on the right is due to 13.7 cm of Hg plus atmospheric pressure.)
\(P_{\mathrm{atmospheric}}\) \(= 1\ \mathrm{atm}\)
\(P_{\mathrm{gas}}\) \(= ΔP + P_{\mathrm{atmospheric}}\)
\(\ \ \ =137.\ \mathrm{mmHg} + 1\ \mathrm{atm}\)
\(\ \ \ =1.180\ \mathrm{atm}\)
\(P_{\mathrm{gas}}\) \(= 1.180\ \mathrm{atm}\)
\(\ \ \ =897.\ \mathrm{mmHg}\)
\(P_{\mathrm{gas}}\) \(= 897.\ \mathrm{mmHg}\)
\(\ \ \ =1.180\ \mathrm{atm}\)
\(P_{\mathrm{gas}}\) \(= 1.180\ \mathrm{atm}\)
\(\ \ \ =119.6\ \mathrm{kPa}\)